Korean J Ophthalmol.
2019 Jun;33(3):259-266. 10.3341/kjo.2018.0106.
Intravitreal Dexamethasone Implantation in Intravitreal Bevacizumab Treatment-resistant Pseudophakic Cystoid Macular Edema
- Affiliations
-
- 1Department of Ophthalmology, Ankara Ulucanlar Eye Education and Research Hospital, University of Health Sciences, Ankara, Turkey. aysegulkaltintas@hotmail.com
- 2Department of Ophthalmology, Hatay State Hospital, Hatay, Turkey.
- KMID: 2448865
- DOI: http://doi.org/10.3341/kjo.2018.0106
Abstract
- PURPOSE
To evaluate the changes in visual acuity (VA) and central macular thickness (CMT) after intravitreal dexamethasone (IVD) implantation in intravitreal bevacizumab (IVB) treatment-resistant cases with pseudophakic cystoid macular edema (PCME).
METHODS
This study included 10 PCME cases who underwent uneventful phacoemulsification and intraocular lens implantation with similar methods and six PCME cases referred to our hospital for treatment of low VA after cataract surgery. Due to the persistence of PCME, both topical steroid and anti-inflammatory medication were administered first, followed by IVB injection. IVD implantation was performed for all IVB treatment-resistant cases. VA and CMT values were compared before and at three months after the first IVD implantation.
RESULTS
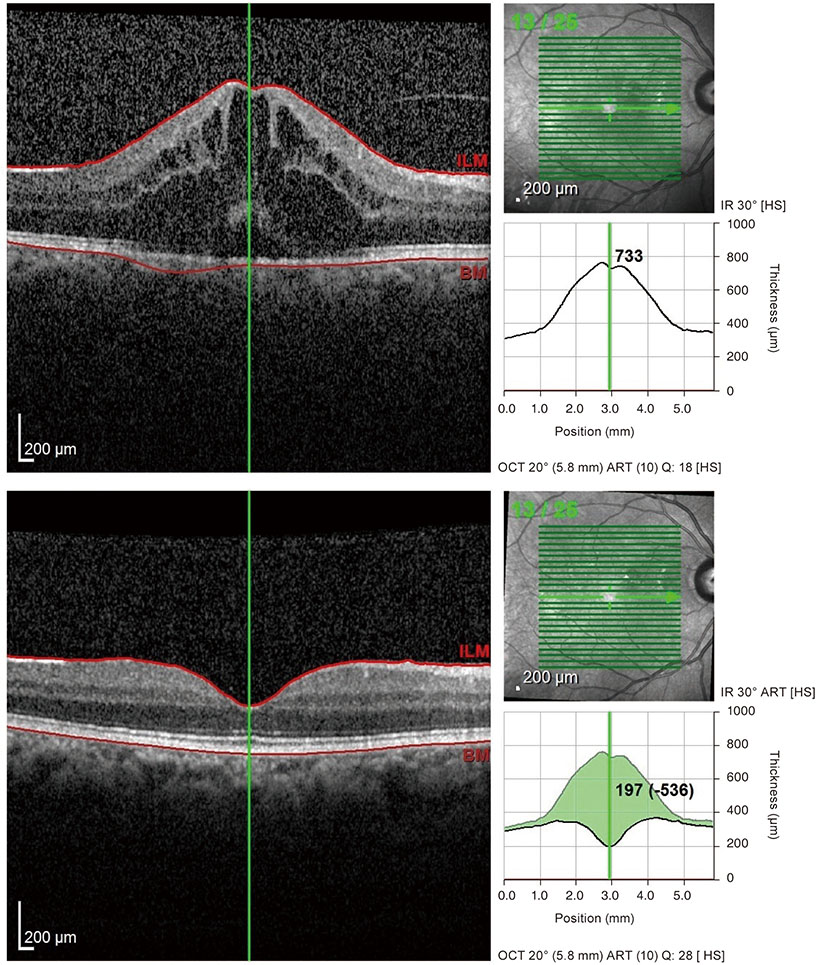
The mean VA values before and at 3 months after the first IVD implantation were 0.69 ± 0.19 logarithm of the minimum angle of resolution (logMAR) (1.50 to 0.10 logMAR) and 0.26 ± 0.07 logMAR (1.00 to 0.00 logMAR), respectively (p < 0.001). The mean CMT was 476.13 ± 135.13 mm (314 to 750 mm) and 294.06 ± 15.26 mm (222 to 480 mm), respectively (p < 0.001). The mean number of implanted IVD was 1.44 ± 0.89 (1 to 4) and the mean follow-up time was 7.4 ± 4.6 months (6 to 24 months). After IVD implantation therapy, the mean VA and CMT values were 0.19 ± 0.05 logMAR (0.70 to 0.00 logMAR) and 268.38 ± 31.35 mm (217 to 351 mm), respectively.
CONCLUSIONS
To the best of our knowledge, this is the first report to show the efficacy of IVD implantation even after repeated IVB injections in treatment-resistant PCME. IVD implantation is both a safe and effective method for decreasing PCME after both uneventful and complicated cataract surgery.
MeSH Terms
Figure
Reference
-
1. Yonekawa Y, Kim IK. Pseudophakic cystoid macular edema. Curr Opin Ophthalmol. 2012; 23:26–32.
Article2. Gass JD, Norton EW. Cystoid macular edema and papilledema following cataract extraction: a fluorescein fundoscopic and angiographic study. Arch Ophthalmol. 1966; 76:646–661.3. Schubert HD. Cystoid macular edema: the apparent role of mechanical factors. Prog Clin Biol Res. 1989; 312:277–291.4. Ursell PG, Spalton DJ, Whitcup SM, Nussenblatt RB. Cystoid macular edema after phacoemulsification: relationship to blood-aqueous barrier damage and visual acuity. J Cataract Refract Surg. 1999; 25:1492–1497.
Article5. Henderson BA, Kim JY, Ament CS, et al. Clinical pseudophakic cystoid macular edema. Risk factors for development and duration after treatment. J Cataract Refract Surg. 2007; 33:1550–1558.6. Eriksson U, Alm A, Bjarnhall G, et al. Macular edema and visual outcome following cataract surgery in patients with diabetic retinopathy and controls. Graefes Arch Clin Exp Ophthalmol. 2011; 249:349–359.
Article7. Chu CJ, Johnston RL, Buscombe C, et al. Risk factors and incidence of macular edema after cataract surgery: a database study of 81984 eyes. Ophthalmology. 2016; 123:316–323.8. Sacconi R, Corbelli E, Carnevali A, et al. Optical coherence tomography angiography in pseudophakic cystoid macular oedema compared to diabetic macular oedema: qualitative and quantitative evaluation of retinal vasculature. Br J Ophthalmol. 2018; 102:1684–1690.
Article9. Benitah NR, Arroyo JG. Pseudophakic cystoid macular edema. Int Ophthalmol Clin. 2010; 50:139–153.
Article10. Vukicevic M, Gin T, Al-Qureshi S. Prevalence of optical coherence tomography-diagnosed postoperative cystoid macular oedema in patients following uncomplicated phaco-emulsification cataract surgery. Clin Exp Ophthalmol. 2012; 40:282–287.
Article11. Altintas AG, Coban P, Arifoglu HB, et al. Comparison of phaco parameters effect on macular thickness changes after uneventful phacosurgery in diabetic and non-diabetic patients. Int Eye Sci. 2016; 16:201–206.12. Lin CJ, Tsai YY. Use of aflibercept for the management of refractory pseudophakic macular edema in Irvine-Gass syndrome and literature review. Retin Cases Brief Rep. 2018; 12:59–62.
Article13. Kiernan DF, Hariprasad SM. Controversies in the management of Irvine-Gass syndrome. Ophthalmic Surg Lasers Imaging Retina. 2013; 44:522–527.
Article14. Randazzo A, Vinciguerra P. Chronic macular edema medical treatment in Irvine-Gass syndrome: case report. Eur J Ophthalmol. 2010; 20:462–465.
Article15. Ilhan C. Current developments in monofocal intraocular lens technology. Int J Ophthalmic Res. 2017; 3:239–242.16. Lobo CL, Faria PM, Soares MA, et al. Macular alterations after small-incision cataract surgery. J Cataract Refract Surg. 2004; 30:752–760.
Article17. Hudes GR, Li WY, Rockey JH, White P. Prostacyclin is the major prostaglandin synthesized by bovine retinal capillary pericytes in culture. Invest Ophthalmol Vis Sci. 1988; 29:1511–1516.18. Shelsta HN, Jampol LM. Pharmacologic therapy of pseudophakic cystoid macular edema: 2010 update. Retina. 2011; 31:4–12.19. Brynskov T, Laugesen CS, Halborg J, et al. Longstanding refractory pseudophakic cystoid macular edema resolved using intravitreal 0.7 mg dexamethasone implants. Clin Ophthalmol. 2013; 7:1171–1174.
Article20. Dang Y, Mu Y, Li L, et al. Comparison of dexamethasone intravitreal implant and intravitreal triamcinolone acetonide for the treatment of pseudophakic cystoid macular edema in diabetic patients. Drug Des Devel Ther. 2014; 8:1441–1449.
Article21. Garcia JM, Isaac DL, Avila MP. Dexamethasone 0.7 mg implants in the management of pseudophakic cystoid macular edema. Arq Bras Oftalmol. 2016; 79:113–115.
Article22. Kakkassery V, Schultz T, Wunderlich MI, et al. Evaluation of predictive factors for successful intravitreal dexamethasone in pseudophakic cystoid macular edema. J Ophthalmol. 2017; 2017:4625730.
Article23. Mayer WJ, Kurz S, Wolf A, et al. Dexamethasone implant as an effective treatment option for macular edema due to Irvine-Gass syndrome. J Cataract Refract Surg. 2015; 41:1954–1961.
Article24. Dutra Medeiros M, Navarro R, Garcia-Arumi J, et al. Dexamethasone intravitreal implant for treatment of patients with recalcitrant macular edema resulting from Irvine-Gass syndrome. Invest Ophthalmol Vis Sci. 2013; 54:3320–3324.
Article25. Tolentino MJ, McLeod DS, Taomoto M, et al. Pathologic features of vascular endothelial growth factor-induced retinopathy in the nonhuman primate. Am J Ophthalmol. 2002; 133:373–385.
Article26. Arevalo JF, Maia M, Garcia-Amaris RA, et al. Intravitreal bevacizumab for refractory pseudophakic cystoid macular edema: the Pan-American Collaborative Retina Study Group results. Ophthalmology. 2009; 116:1481–1487.27. Mason JO 3rd, Albert MA Jr, Vail R. Intravitreal bevacizumab (Avastin) for refractory pseudophakic cystoid macular edema. Retina. 2006; 26:356–357.
Article28. Barone A, Prascina F, Russo V, et al. Successful treatment of pseudophakic cystoid macular edema with intravitreal bevacizumab. J Cataract Refract Surg. 2008; 34:1210–1212.
Article29. Spitzer MS, Ziemssen F, Yoeruek E, et al. Efficacy of intravitreal bevacizumab in treating postoperative pseudophakic cystoid macular edema. J Cataract Refract Surg. 2008; 34:70–75.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Repeated Intravitreal Dexamethasone Implantation for Treatment of Macular Edema after Scleral Fixation of Intraocular Lens
- A Case of Retinal Hemorrhage Following a Dexamethasone Intravitreal Implant
- Microaneurysm Turnover after the Use of Dexamethasone and Bevacizumab to Treat Diabetic Macular Edema
- The Effects of Intravitreal Bevacizumab Injection According to the Type of Diabetic Macular Edema
- Short-Term Results of Dexamethasone Intravitreal Implant in Patients with Refractory Diabetic Macular Edema