Korean J Ophthalmol.
2019 Jun;33(3):238-248. 10.3341/kjo.2018.0130.
Comparison of Retinal Layer Thickness and Vascular Density between Acute and Chronic Branch Retinal Vein Occlusion
- Affiliations
-
- 1Department of Ophthalmology, Korea University College of Medicine, Seoul, Korea.
- 2Department of Ophthalmology, CHA Bundang Medical Center, CHA University, Seongnam, Korea. ja01@cha.ac.kr
- KMID: 2448863
- DOI: http://doi.org/10.3341/kjo.2018.0130
Abstract
- PURPOSE
To compare retinal layer thickness and chorioretinal vascular density (VD) between acute and chronic branch retinal vein occlusion (BRVO).
METHODS
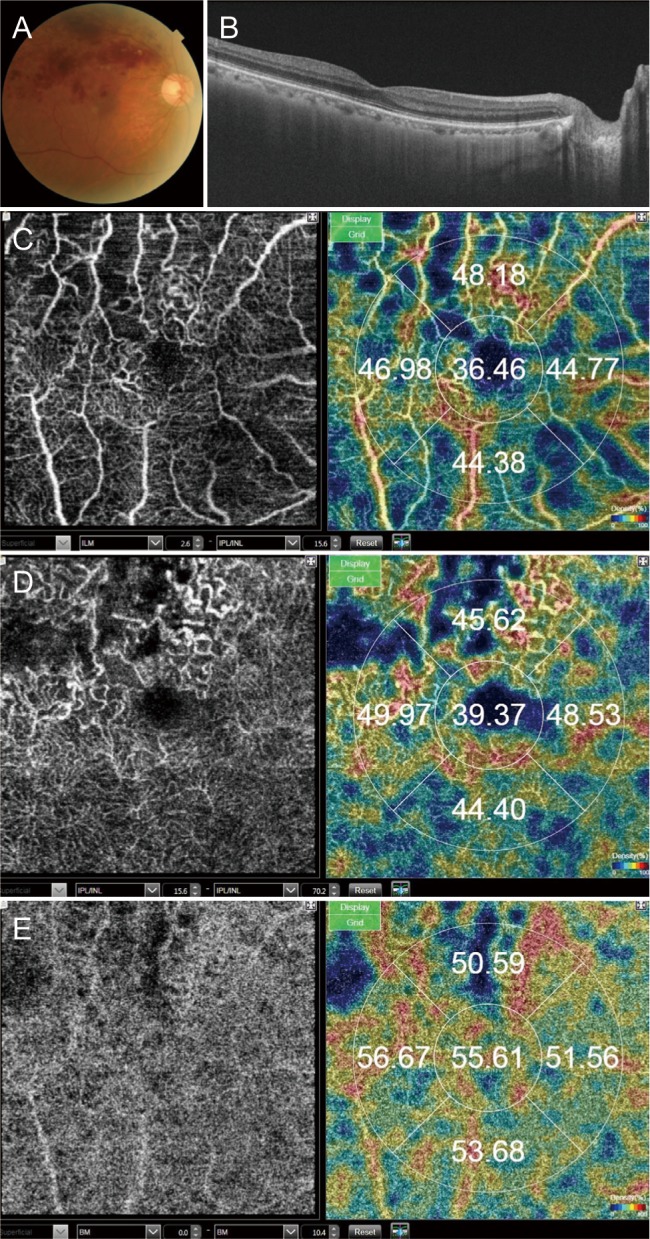
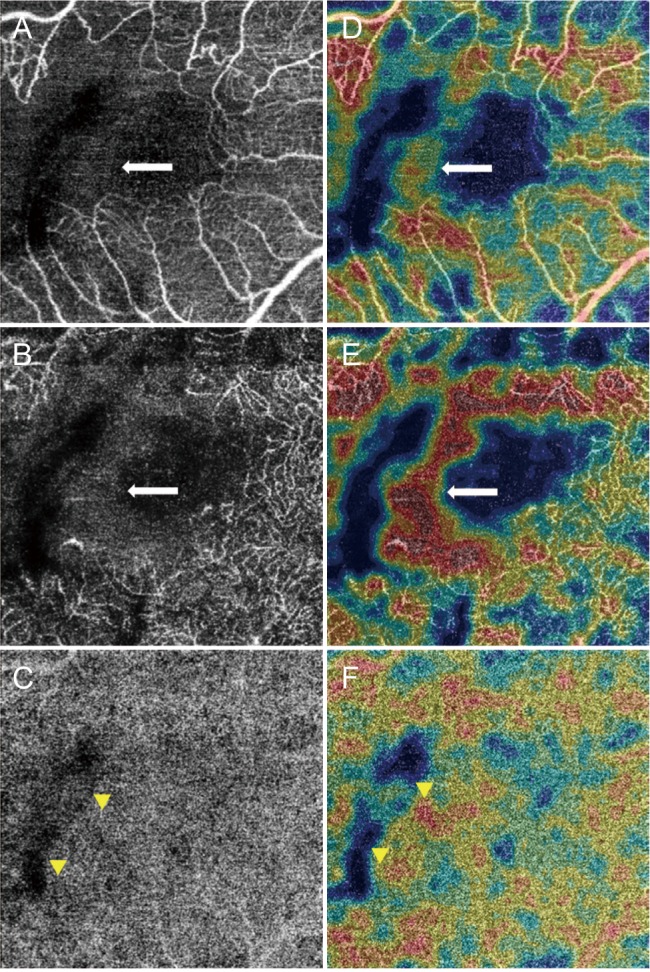
This study included patients with BRVO. The VD of the superficial capillary plexus (VDs), the VD of the deep capillary plexus (VDd), and VD of the choriocapillaris were obtained using optical coherence tomography angiography. Acute and chronic BRVO data were compared to assess differences between the involved and uninvolved areas.
RESULTS
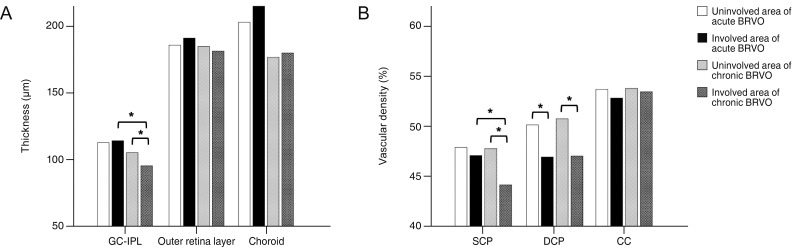
We included 17 eyes with acute BRVO and 23 eyes with chronic BRVO. The VDs in the involved area were not significantly different between the involved area and in the uninvolved area in acute BRVO (p = 0.551). However, the difference was significant in chronic BRVO (p = 0.013). The VDd in the involved area was lower than in the uninvolved area in both acute and chronic BRVO (p = 0.020, p = 0.003, respectively). In addition, the VD of the choriocapillaris values did not differ significantly between acute and chronic BRVO, or between involved and uninvolved areas. The VDs in the involved area in chronic BRVO were lower than in acute BRVO (p = 0.047), and the VDd did not differ between acute and chronic BRVO in all areas.
CONCLUSIONS
Vascular impaired patterns in the retinal layer differed between acute and chronic BRVO. These results may suggest that vascular change and remodeling develops differently in acute and chronic phases in BRVO.
Keyword
MeSH Terms
Figure
Reference
-
1. Huang Y, Zhang Q, Thorell MR, et al. Swept-source OCT angiography of the retinal vasculature using intensity differentiation-based optical microangiography algorithms. Ophthalmic Surg Lasers Imaging Retina. 2014; 45:382–389. PMID: 25230403.
Article2. Kuehlewein L, Bansal M, Lenis TL, et al. Optical coherence tomography angiography of type 1 neovascularization in age-related macular degeneration. Am J Ophthalmol. 2015; 160:739–748. PMID: 26164826.3. El Ameen A, Cohen SY, Semoun O, et al. Type 2 neovascularization secondary to age-related macular degeneration imaged by optical coherence tomography angiography. Retina. 2015; 35:2212–2218. PMID: 26441269.
Article4. Miere A, Querques G, Semoun O, et al. Optical coherence tomography angiography in early type 3 neovascularization. Retina. 2015; 35:2236–2241. PMID: 26457399.
Article5. Hwang TS, Jia Y, Gao SS, et al. Optical coherence tomography angiography features of diabetic retinopathy. Retina. 2015; 35:2371–2376. PMID: 26308529.
Article6. Zhang Q, Wang RK, Chen CL, et al. Swept source optical coherence tomography angiography of neovascular macular telangiectasia type 2. Retina. 2015; 35:2285–2299. PMID: 26457402.
Article7. Jaulim A, Ahmed B, Khanam T, Chatziralli IP. Branch retinal vein occlusion: epidemiology, pathogenesis, risk factors, clinical features, diagnosis, and complications. An update of the literature. Retina. 2013; 33:901–910. PMID: 23609064.8. Chung CY, Tang HH, Li SH, Li KK. Differential microvascular assessment of retinal vein occlusion with coherence tomography angiography and fluorescein angiography: a blinded comparative study. Int Ophthalmol. 2018; 38:1119–1128. PMID: 28550346.
Article9. Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs: an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology. 1991; 98(5 Suppl):786–806. PMID: 2062513.10. Gherghel D, Orgul S, Gugleta K, et al. Relationship between ocular perfusion pressure and retrobulbar blood flow in patients with glaucoma with progressive damage. Am J Ophthalmol. 2000; 130:597–605. PMID: 11078838.
Article11. Chen FK, Viljoen RD, Bukowska DM. Classification of image artefacts in optical coherence tomography angiography of the choroid in macular diseases. Clin Exp Ophthalmol. 2016; 44:388–399. PMID: 26584465.
Article12. Coscas F, Glacet-Bernard A, Miere A, et al. Optical coherence tomography angiography in retinal vein occlusion: evaluation of superficial and deep capillary plexa. Am J Ophthalmol. 2016; 161:160–171. PMID: 26476211.13. Kashani AH, Lee SY, Moshfeghi A, et al. Optical coherence tomography angiography of retinal venous occlusion. Retina. 2015; 35:2323–2331. PMID: 26457395.
Article14. Philippakis E, Dupas B, Bonnin P, et al. Optical coherence tomography angiography shows deep capillary plexus hypoperfusion in incomplete central retinal artery occlusion. Retin Cases Brief Rep. 2015; 9:333–338. PMID: 26355822.
Article15. Scarinci F, Nesper PL, Fawzi AA. Deep retinal capillary nonperfusion is associated with photoreceptor disruption in diabetic macular ischemia. Am J Ophthalmol. 2016; 168:129–138. PMID: 27173374.
Article16. Kang JW, Yoo R, Jo YH, Kim HC. Correlation of microvascular structures on optical coherence tomography angiography with visual acuity in retinal vein occlusion. Retina. 2017; 37:1700–1709. PMID: 27828907.
Article17. Wakabayashi T, Sato T, Hara-Ueno C, et al. Retinal microvasculature and visual acuity in eyes with branch retinal vein occlusion: imaging analysis by optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2017; 58:2087–2094. PMID: 28388705.
Article18. Birol G, Wang S, Budzynski E, et al. Oxygen distribution and consumption in the macaque retina. Am J Physiol Heart Circ Physiol. 2007; 293:H1696–H1704. PMID: 17557923.
Article19. Salthouse TA, Hancock HE, Meinz EJ, Hambrick DZ. Interrelations of age, visual acuity, and cognitive functioning. J Gerontol B Psychol Sci Soc Sci. 1996; 51:P317–P330. PMID: 8931619.
Article20. Pitts DG. Visual acuity as a function of age. J Am Optom Assoc. 1982; 53:117–124. PMID: 7069103.21. Gittings NS, Fozard JL. Age related changes in visual acuity. Exp Gerontol. 1986; 21:423–433. PMID: 3493168.
Article22. Scialfa CT, Cordazzo S, Bubric K, Lyon J. Aging and visual crowding. J Gerontol B Psychol Sci Soc Sci. 2013; 68:522–528. PMID: 23009956.
Article23. Adams AJ, Wong LS, Wong L, Gould B. Visual acuity changes with age: some new perspectives. Am J Optom Physiol Opt. 1988; 65:403–406. PMID: 3407728.24. Collins MJ, Brown B, Bowman KJ. Peripheral visual acuity and age. Ophthalmic Physiol Opt. 1989; 9:314–316. PMID: 2622675.
Article25. Lim HB, Kim MS, Jo YJ, Kim JY. Prediction of retinal ischemia in branch retinal vein occlusion: spectral-domain optical coherence tomography study. Invest Ophthalmol Vis Sci. 2015; 56:6622–6629. PMID: 26465891.
Article26. Kim CS, Shin KS, Lee HJ, et al. Sectoral retinal nerve fiber layer thinning in branch retinal vein occlusion. Retina. 2014; 34:525–530. PMID: 23958844.
Article27. Lee J, Moon BG, Cho AR, Yoon YH. Optical coherence tomography angiography of DME and its association with anti-VEGF treatment response. Ophthalmology. 2016; 123:2368–2375. PMID: 27613201.
Article28. Spaide RF. Retinal vascular cystoid macular edema: review and new theory. Retina. 2016; 36:1823–1842. PMID: 27328171.29. Aiello LP, Northrup JM, Keyt BA, et al. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol. 1995; 113:1538–1544. PMID: 7487623.
Article30. Tilton RG, Chang KC, LeJeune WS, et al. Role for nitric oxide in the hyperpermeability and hemodynamic changes induced by intravenous VEGF. Invest Ophthalmol Vis Sci. 1999; 40:689–696. PMID: 10067972.31. Kim KH, Lee DH, Lee JJ, et al. Regional choroidal thickness changes in branch retinal vein occlusion with macular edema. Ophthalmologica. 2015; 234:109–118. PMID: 26305536.
Article32. Shin YU, Lee MJ, Lee BR. Choroidal maps in different types of macular edema in branch retinal vein occlusion using swept-source optical coherence tomography. Am J Ophthalmol. 2015; 160:328–334. PMID: 25959899.
Article33. Du KF, Xu L, Shao L, et al. Subfoveal choroidal thickness in retinal vein occlusion. Ophthalmology. 2013; 120:2749–2750. PMID: 24246829.
Article34. Rishi P, Rishi E, Mathur G, Raval V. Ocular perfusion pressure and choroidal thickness in eyes with polypoidal choroidal vasculopathy, wet-age-related macular degeneration, and normals. Eye (Lond). 2013; 27:1038–1043. PMID: 23764988.
Article35. Yun C, Ahn J, Kim M, et al. Ocular perfusion pressure and choroidal thickness in early age-related macular degeneration patients with reticular pseudodrusen. Invest Ophthalmol Vis Sci. 2016; 57:6604–6609. PMID: 27926751.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Monocytes and High-density Lipoprotein Cholesterol in Branch Retinal Vein Occlusion
- Clinical Features According to the Occlusion Site in Patients with Branch Retinal Vein Occlusion
- The Relationship between the Capillary Nonperfusion Area and the Retinal Nerve Fiber Layer Defects in Branch Retinal Vein Occlusion Patients
- Clinical observation of the bilateral branch vein occlusion
- Eales Disease Accompanied with Branch Retinal Vein Occlusion