Int J Stem Cells.
2019 Mar;12(1):43-50. 10.15283/ijsc18056.
Incidence, Risk Factors and Prognosis of Acute Kidney Injury Following Hematopoietic Stem Cell Transplant: A Pilot Study
- Affiliations
-
- 1Department of Clinical Nursing, The University of Jordan, Amman, Jordan.
- 2Department of Nursing, Jordan University Hospital, Amman, Jordan.
- 3Faculty of Medicine and Cell Therapy Center, Jordan University Hospital, Amman, Jordan. abdalla.awidi@gmail.com
- KMID: 2447223
- DOI: http://doi.org/10.15283/ijsc18056
Abstract
- BACKGROUND AND OBJECTIVES
The burden of acute kidney injury (AKI) has not been explored in Jordanian patients who receive hematopoietic stem cell transplant (HSCT). The aim of this study was to evaluate the frequency, risk factors, and mortality of AKI among patients who underwent HSCT.
METHODS
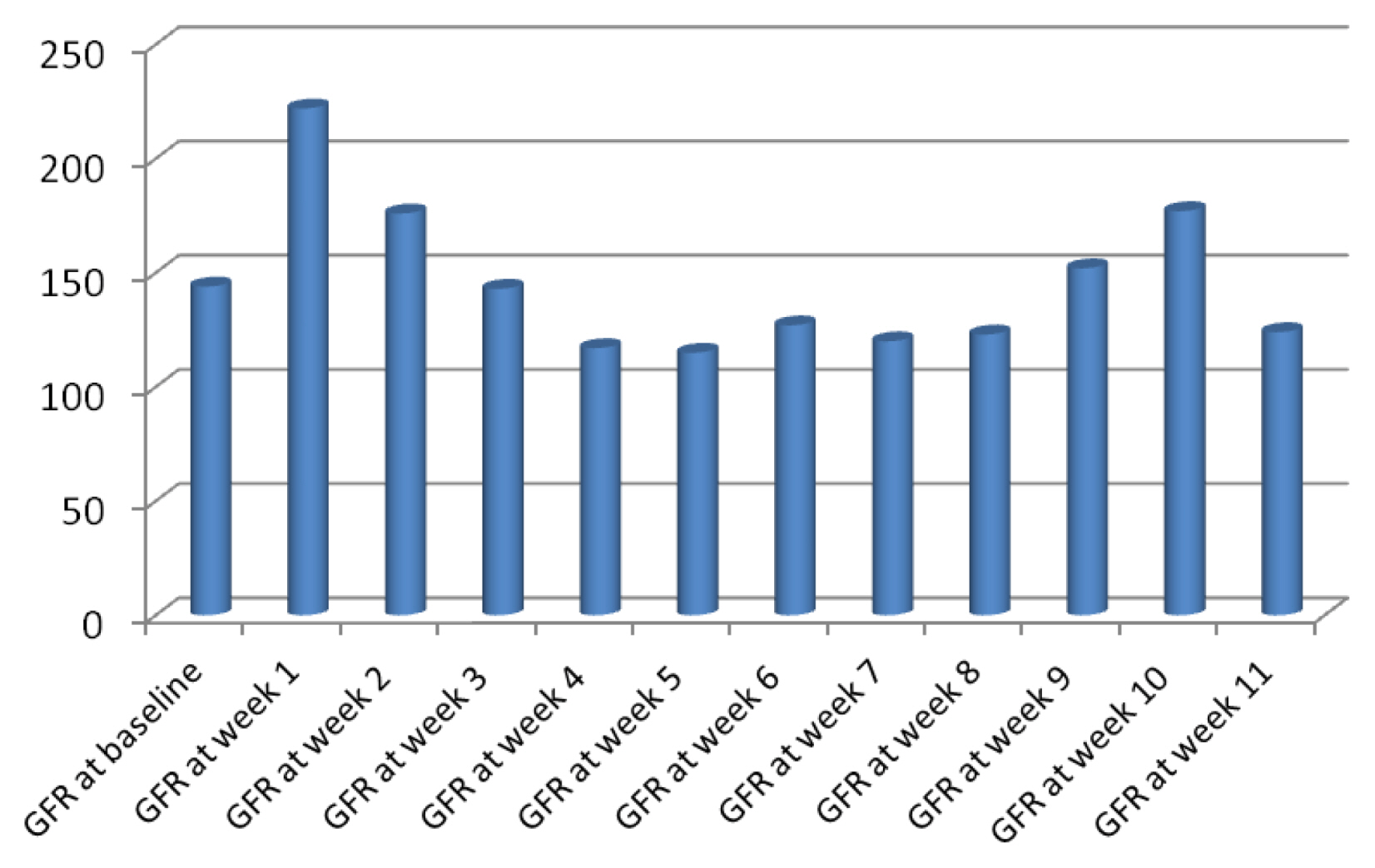
A retrospective pilot study included 70 adult patients who received peripheral HSCT was conducted. Weekly measurement of serum creatinine (SCr) was obtained for 3 months after chemotherapy and HSCT. Then, stages of Risk, Injury, and Failure of Kidney were determined based on the Kidney Disease for Improving Global Outcomes (KDIGO).
RESULTS
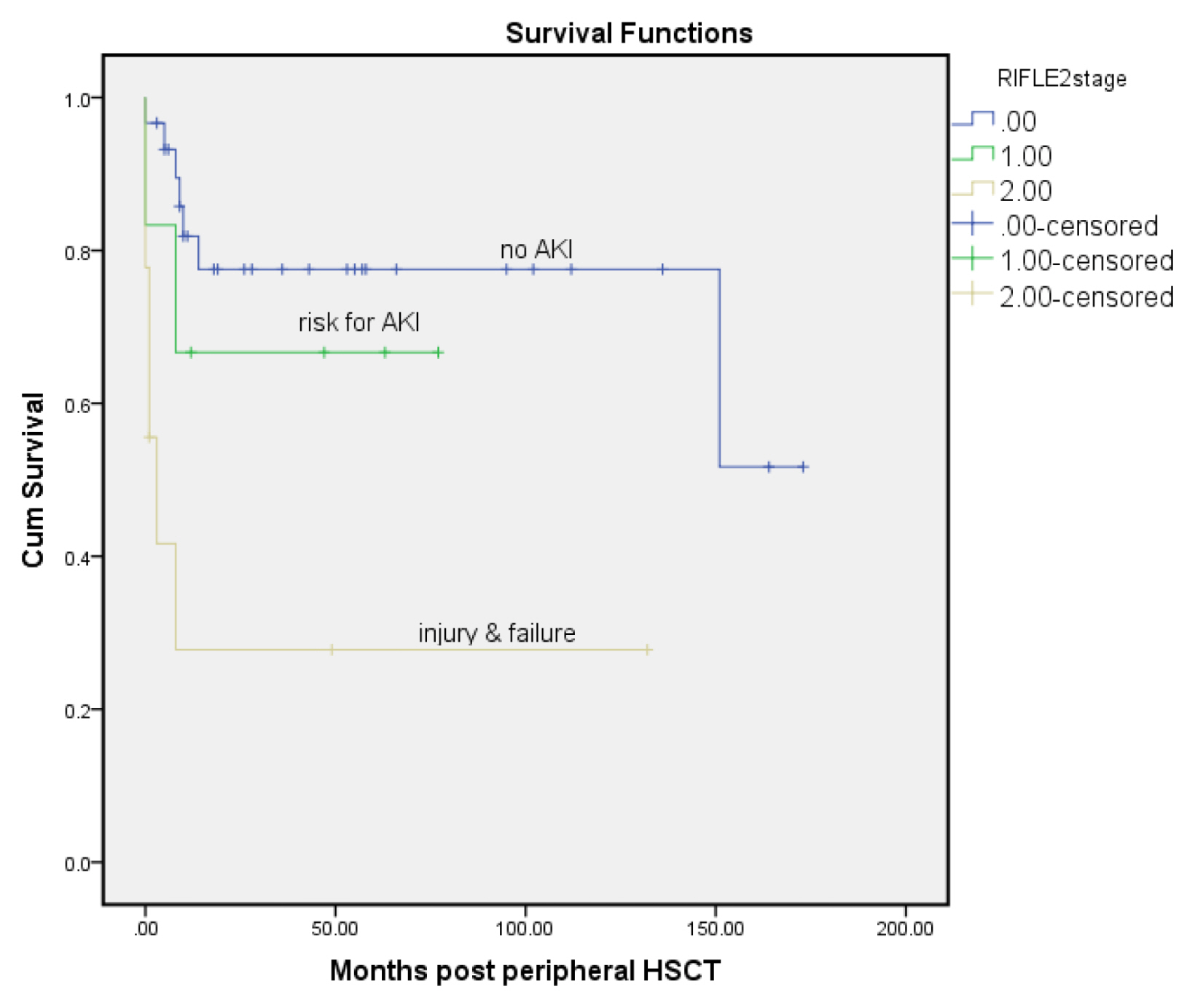
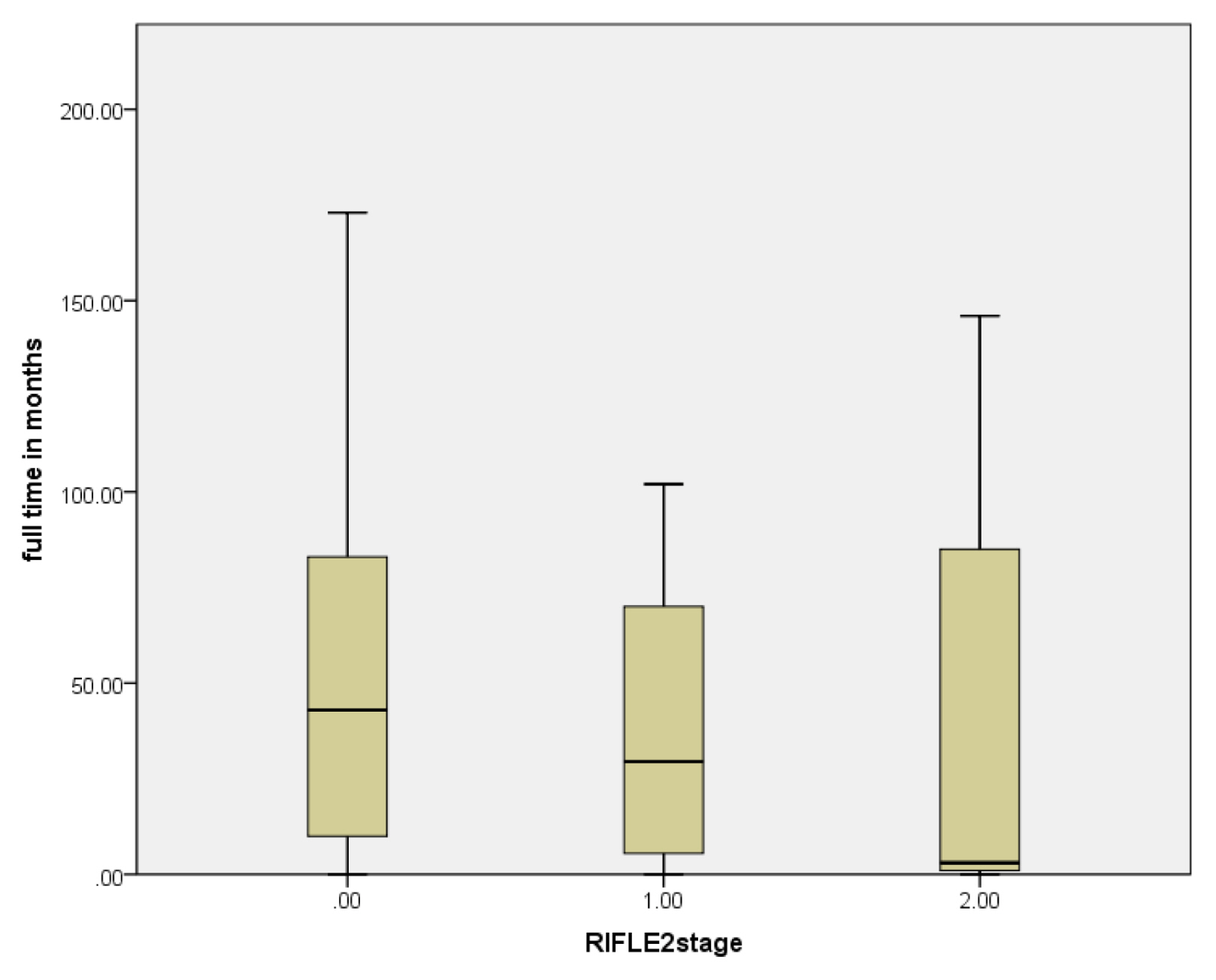
The median follow-up was 41 months. Mortality was reported in 16 patients (23%). Out of 60 patients that had SCr values, 19 patients (31.6%) had AKI in 90 days after chemotherapy. Allogeneic HSCT, male donors, high-dose melphalan protocols and values of blood urea nitrogen (BUN) were significantly higher among patients with AKI.
CONCLUSIONS
Combining many nephrotoxic drugs and dosing adjustments should be considered in uniform protocols. Multidisciplinary care should be utilized to assess early kidney dysfunction that decreases adverse events and improves outcomes.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Ljungman P, Bregni M, Brune M, Cornelissen J, de Witte T, Dini G, Einsele H, Gaspar HB, Gratwohl A, Passweg J, Peters C, Rocha V, Saccardi R, Schouten H, Sureda A, Tichelli A, Velardi A, Niederwieser D. European Group for Blood and Marrow Transplantation. Allogeneic and autologous transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe 2009. Bone Marrow Transplant. 2010; 45:219–234. DOI: 10.1038/bmt.2009.141. PMID: 19584824.
Article2. Majhail NS, Farnia SH, Carpenter PA, Champlin RE, Crawford S, Marks DI, Omel JL, Orchard PJ, Palmer J, Saber W, Savani BN, Veys PA, Bredeson CN, Giralt SA, LeMaistre CF. Indications for autologous and allogeneic hematopoietic cell transplantation: guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2015; 21:1863–1869. DOI: 10.1016/j.bbmt.2015.07.032. PMID: 26256941. PMCID: 4830270.
Article3. Ljungman P, Gratwohl A. Indication and current practice. Ljungman P, Gratwohl A, editors. Indications and current practice for allogeneic and autologous HSCT for haematological diseases, solid tumors and immune disorders 2008. Barcelona: European Group for Blood and Marrow Transplantation.4. Amayiri N, Al-Zaben A, Ghatasheh L, Frangoul H, Hussein AA. Hematopoietic stem cell transplantation for children with primary immunodeficiency diseases: single center experience in Jordan. Pediatr Transplant. 2013; 17:394–402. DOI: 10.1111/petr.12081. PMID: 23692601.
Article5. Aladily TN, Mohammad RS, Al-Khader A, Awidi AS. Essential thrombocythemia in a two-year-old child, responsive to hydroxyurea but not aspirin. Oman Med J. 2017; 32:243–246. DOI: 10.5001/omj.2017.45. PMID: 28584607. PMCID: 5447791.
Article6. Alaloul F, Brockopp DY, Andrykowski MA, Hall LA, Al Nusairat TS. Quality of life in Arab Muslim cancer survivors following hematopoietic stem cell transplantation: comparison with matched healthy group. Support Care Cancer. 2015; 23:2157–2164. DOI: 10.1007/s00520-014-2583-7. PMID: 25556704.
Article7. Alaloul F, Schreiber JA, Al Nusairat TS, Andrykowski MA. Spirituality in Arab Muslim hematopoietic stem cell transplantation survivors: a qualitative approach. Cancer Nurs. 2016; 39:E39–47. DOI: 10.1097/NCC.0000000000000312. PMID: 26474217.8. Bird JM, Fuge R, Sirohi B, Apperley JF, Hunter A, Snowden J, Mahendra P, Milligan D, Byrne J, Littlewood T, Fegan C, McQuaker G, Pagliuca A, Johnson P, Rahemtulla A, Morris C, Marks DI. British Society of Blood and Marrow Transplantation. The clinical outcome and toxicity of high-dose chemotherapy and autologous stem cell transplantation in patients with myeloma or amyloid and severe renal impairment: a British Society of Blood and Marrow Transplantation study. Br J Haematol. 2006; 134:385–390. DOI: 10.1111/j.1365-2141.2006.06191.x. PMID: 16822294.
Article9. Saddadi F, Najafi I, Hakemi MS, Falaknazi K, Attari F, Bahar B. Frequency, risk factors, and outcome of acute kidney injury following bone marrow transplantation at Dr Shariati Hospital in Tehran. Iran J Kidney Dis. 2010; 4:20–26. PMID: 20081300.10. Liu H, Li YF, Liu BC, Ding JH, Chen BA, Xu WL, Qian J. A multicenter, retrospective study of acute kidney injury in adult patients with nonmyeloablative hematopoietic SCT. Bone Marrow Transplant. 2010; 45:153–158. DOI: 10.1038/bmt.2009.99. PMID: 19430501.
Article11. Lopes JA, Jorge S, Neves M. Acute kidney injury in HCT: an update. Bone Marrow Transplant. 2016; 51:755–762. DOI: 10.1038/bmt.2015.357. PMID: 26855155.
Article12. Shingai N, Morito T, Najima Y, Kobayashi T, Doki N, Kakihana K, Ohashi K, Ando M. Early-onset acute kidney injury is a poor prognostic sign for allogeneic SCT recipients. Bone Marrow Transplant. 2015; 50:1557–1562. DOI: 10.1038/bmt.2015.188. PMID: 26301965.
Article13. Kidney Disease Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012; 2:1–138.14. Aladily TN, Mohammad RS, Al-Khader A, Awidi AS. Essential thrombocythemia in a two-year-old child, responsive to hydroxyurea but not aspirin. Oman Med J. 2017; 32:243–246. DOI: 10.5001/omj.2017.45. PMID: 28584607. PMCID: 5447791.
Article15. Passweg JR, Baldomero H, Bader P, Bonini C, Cesaro S, Dreger P, Duarte RF, Dufour C, Kuball J, Farge-Bancel D, Gennery A, Kröger N, Lanza F, Nagler A, Sureda A, Mohty M. Hematopoietic stem cell transplantation in Europe 2014: more than 40 000 transplants annually. Bone Marrow Transplant. 2016; 51:786–792. DOI: 10.1038/bmt.2016.20. PMID: 26901709. PMCID: 4895175.
Article16. Alramly M, Darawad MW, Khalil AA. Slowing the progression of chronic kidney disease: comparison between predialysis and dialysis in Jordanian patients. Ren Fail. 2013; 35:1348–1352. DOI: 10.3109/0886022X.2013.828260. PMID: 23992491.
Article17. Parikh CR, McSweeney PA, Korular D, Ecder T, Merouani A, Taylor J, Slat-Vasquez V, Shpall EJ, Jones RB, Bearman SI, Schrier RW. Renal dysfunction in allogeneic hematopoietic cell transplantation. Kidney Int. 2002; 62:566–573. DOI: 10.1046/j.1523-1755.2002.00455.x. PMID: 12110019.
Article18. Chow EJ, Cushing-Haugen KL, Cheng GS, Boeckh M, Khera N, Lee SJ, Leisenring WM, Martin PJ, Mueller BA, Schwartz SM, Baker KS. Morbidity and mortality differences between hematopoietic cell transplantation survivors and other cancer survivors. J Clin Oncol. 2017; 35:306–313. DOI: 10.1200/JCO.2016.68.8457. PMID: 27870568. PMCID: 5456375.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Primary renal aspergillosis and renal stones in both kidneys associated with hematopoietic stem cell transplant
- Acute kidney injury in the patient with cancer
- Allogeneic hematopoietic stem cell transplantation for myelodysplastic syndromes
- Acute Renal Failure after Hematopoietic Cell Transplantation : Cause and Prognosis
- Hematopoietic stem cell transplantation: overview for general pediatrician