Anat Cell Biol.
2018 Sep;51(3):180-188. 10.5115/acb.2018.51.3.180.
Stem cell transplantation and functional recovery after spinal cord injury: a systematic review and meta-analysis
- Affiliations
-
- 1Hearing Disorders Research Center, Loghman Hakim Medical Center and Department of Biology and Anatomical Sciences, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
- 2Hearing Disorders Research Center, Loghman Hakim Medical Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
- 3Cellular and Molecular Research Center, Qazvin University of Medical Sciences, Qazvin, Iran.
- 4Department of Clinical Biochemistry, Faculty of Paramedicine, Ilam University of Medical Sciences, Ilam, Iran.
- 5Department of Statistics, University of Qom, Qom, Iran.
- 6Department of Biotechnology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran. dr.ysadeghi@yahoo.com
- 7G. Raymond Chang School, Ryerson University, Toronto, Canada.
- KMID: 2447011
- DOI: http://doi.org/10.5115/acb.2018.51.3.180
Abstract
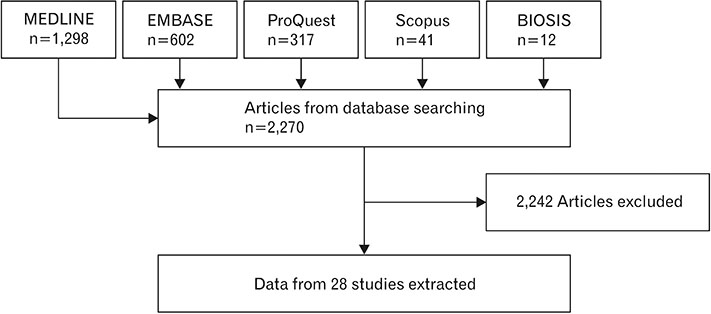
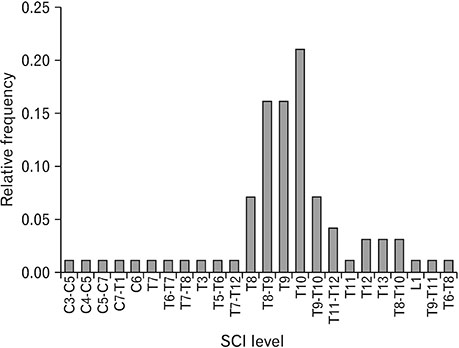
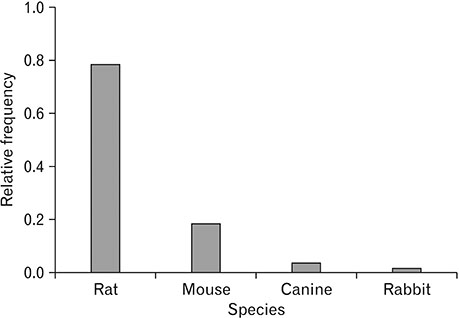
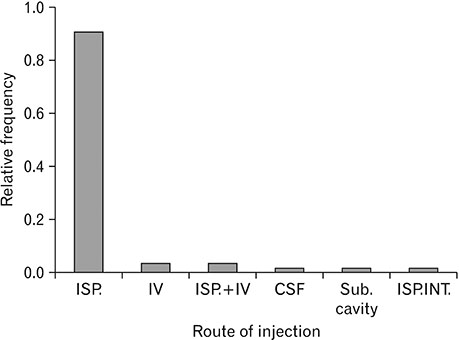
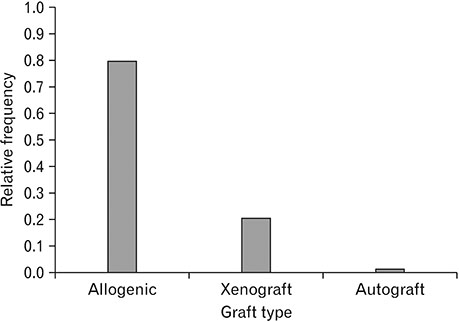
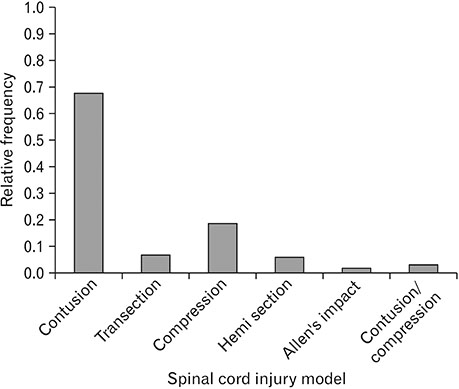
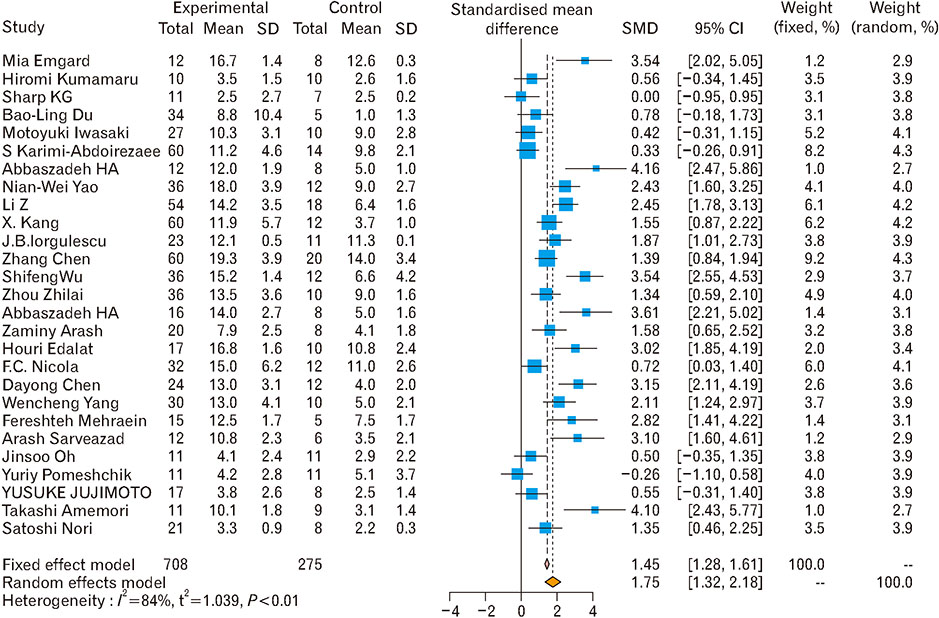
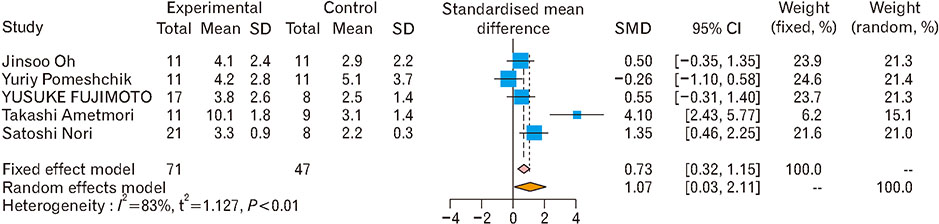
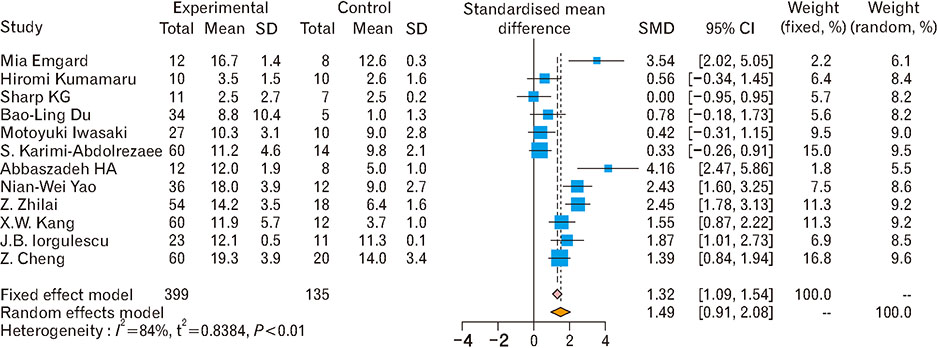
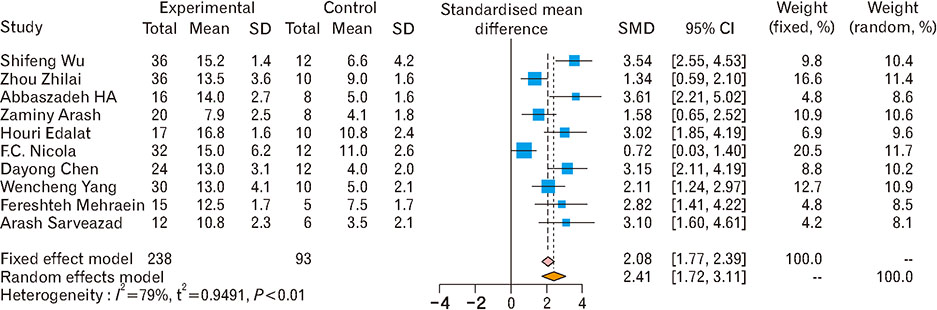
- Spinal cord injury is a significant cause of motor dysfunctions. There is no definite cure for it, and most of the therapeutic modalities are only symptomatic treatment. In this systematic review and meta-analysis, the effectiveness of stem cell therapy in the treatment of the spinal cord injuries in animal models was studied and evaluated. A systematic search through medical databases by using appropriate keywords was conducted. The relevant reports were reviewed in order to find out cases in which inclusion and exclusion criteria had been fulfilled. Finally, 89 articles have been considered, from which 28 had sufficient data for performing statistical analyses. The findings showed a significant improvement in motor functions after cell therapy. The outcome was strongly related to the number of transplanted cells, site of injury, chronicity of the injury, type of the damage, and the induction of immune-suppression. According to our data, improvements in functional recovery after stem cell therapy in the treatment of spinal cord injury in animal models was noticeable, but its outcome is strongly related to the site of injury, number of transplanted cells, and type of transplanted cells.
MeSH Terms
Figure
Cited by 1 articles
-
Ibrutinib reduces neutrophil infiltration, preserves neural tissue and enhances locomotor recovery in mouse contusion model of spinal cord injury
Somayyeh Torabi, Seyed Hadi Anjamrooz, Zahra Zeraatpisheh, Hadi Aligholi, Hassan Azari
Anat Cell Biol. 2021;54(3):350-360. doi: 10.5115/acb.20.299.
Reference
-
1. Kim Y, Kim J, Ahn M, Shin T. Lithium ameliorates rat spinal cord injury by suppressing glycogen synthase kinase-3beta and activating heme oxygenase-1. Anat Cell Biol. 2017; 50:207–213.2. Emgård M, Piao J, Aineskog H, Liu J, Calzarossa C, Odeberg J, Holmberg L, Samuelsson EB, Bezubik B, Vincent PH, Falci SP, Seiger Å, Åkesson E, Sundström E. Neuroprotective effects of human spinal cord-derived neural precursor cells after transplantation to the injured spinal cord. Exp Neurol. 2014; 253:138–145.
Article3. Wu S, Cui G, Shao H, Du Z, Ng JC, Peng C. The cotransplantation of olfactory ensheathing cells with bone marrow mesenchymal stem cells exerts antiapoptotic effects in adult rats after spinal cord injury. Stem Cells Int. 2015; 2015:516215.
Article4. Kumamaru H, Saiwai H, Kubota K, Kobayakawa K, Yokota K, Ohkawa Y, Shiba K, Iwamoto Y, Okada S. Therapeutic activities of engrafted neural stem/precursor cells are not dormant in the chronically injured spinal cord. Stem Cells. 2013; 31:1535–1547.
Article5. Sharp KG, Yee KM, Steward O. A re-assessment of long distance growth and connectivity of neural stem cells after severe spinal cord injury. Exp Neurol. 2014; 257:186–204.
Article6. Oh J, Lee KI, Kim HT, You Y, Yoon DH, Song KY, Cheong E, Ha Y, Hwang DY. Human-induced pluripotent stem cells generated from intervertebral disc cells improve neurologic functions in spinal cord injury. Stem Cell Res Ther. 2015; 6:125.
Article7. Pomeshchik Y, Puttonen KA, Kidin I, Ruponen M, Lehtonen S, Malm T, Åkesson E, Hovatta O, Koistinaho J. Transplanted human induced pluripotent stem cell-derived neural progenitor cells do not promote functional recovery of pharmacologically immunosuppressed mice with contusion spinal cord injury. Cell Transplant. 2015; 24:1799–1812.
Article8. Nguyen HX, Beck KD, Anderson AJ. Quantitative assessment of immune cells in the injured spinal cord tissue by flow cytometry: a novel use for a cell purification method. J Vis Exp. 2011; (50):2698.
Article9. Wei GJ, An G, Shi ZW, Wang KF, Guan Y, Wang YS, Han B, Yu EM, Li PF, Dong DM, Wang LP, Teng ZW, Zhao DL. Suppression of microRNA-383 enhances therapeutic potential of human bone-marrow-derived mesenchymal stem cells in treating spinal cord injury via GDNF. Cell Physiol Biochem. 2017; 41:1435–1444.
Article10. Abbaszadeh HA, Tiraihi T, Delshad A, Saghedizadeh M, Taheri T, Kazemi H, Hassoun HK. Differentiation of neurosphere-derived rat neural stem cells into oligodendrocyte-like cells by repressing PDGF-alpha and Olig2 with triiodothyronine. Tissue Cell. 2014; 46:462–469.11. Abbaszadeh HA, Tiraihi T, Delshad AR, Saghedi Zadeh M, Taheri T. Bone marrow stromal cell transdifferentiation into oligodendrocyte-like cells using triiodothyronine as a inducer with expression of platelet-derived growth factor alpha as a maturity marker. Iran Biomed J. 2013; 17:62–70.12. Li X, Zhao Y, Cheng S, Han S, Shu M, Chen B, Chen X, Tang F, Wang N, Tu Y, Wang B, Xiao Z, Zhang S, Dai J. Cetuximab modified collagen scaffold directs neurogenesis of injury-activated endogenous neural stem cells for acute spinal cord injury repair. Biomaterials. 2017; 137:73–86.
Article13. Morita T, Sasaki M, Kataoka-Sasaki Y, Nakazaki M, Nagahama H, Oka S, Oshigiri T, Takebayashi T, Yamashita T, Kocsis JD, Honmou O. Intravenous infusion of mesenchymal stem cells promotes functional recovery in a model of chronic spinal cord injury. Neuroscience. 2016; 335:221–231.
Article14. Zhilai Z, Biling M, Sujun Q, Chao D, Benchao S, Shuai H, Shun Y, Hui Z. Preconditioning in lowered oxygen enhances the therapeutic potential of human umbilical mesenchymal stem cells in a rat model of spinal cord injury. Brain Res. 2016; 1642:426–435.
Article15. Yang W, Yang Y, Yang JY, Liang M, Song J. Treatment with bone marrow mesenchymal stem cells combined with plumbagin alleviates spinal cord injury by affecting oxidative stress, inflammation, apoptotis and the activation of the Nrf2 pathway. Int J Mol Med. 2016; 37:1075–1082.
Article16. Sistrom CL, Mergo PJ. A simple method for obtaining original data from published graphs and plots. AJR Am J Roentgenol. 2000; 174:1241–1244.
Article17. Iwasaki M, Wilcox JT, Nishimura Y, Zweckberger K, Suzuki H, Wang J, Liu Y, Karadimas SK, Fehlings MG. Synergistic effects of self-assembling peptide and neural stem/progenitor cells to promote tissue repair and forelimb functional recovery in cervical spinal cord injury. Biomaterials. 2014; 35:2617–2629.18. Fujimoto Y, Abematsu M, Falk A, Tsujimura K, Sanosaka T, Juliandi B, Semi K, Namihira M, Komiya S, Smith A, Nakashima K. Treatment of a mouse model of spinal cord injury by transplantation of human induced pluripotent stem cell-derived long-term self-renewing neuroepithelial-like stem cells. Stem Cells. 2012; 30:1163–1173.
Article19. Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Schut D, Fehlings MG. Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J Neurosci. 2010; 30:1657–1676.
Article20. Yao NW, Lu Y, Shi LQ, Xu F, Cai XH. Neuroprotective effect of combining tanshinone IIA with low-dose methylprednisolone following acute spinal cord injury in rats. Exp Ther Med. 2017; 13:2193–2202.
Article21. Li Z, Zhao W, Liu W, Zhou Y, Jia J, Yang L. Transplantation of placenta-derived mesenchymal stem cell-induced neural stem cells to treat spinal cord injury. Neural Regen Res. 2014; 9:2197–2204.
Article22. Kang XW, Hu JL, Wang SK, Wang J. Effectiveness of muscle basal lamina carrying neural stem cells and olfactory ensheathing cells in spinal cord repair. Genet Mol Res. 2015; 14:13437–13455.
Article23. Iorgulescu JB, Patel SP, Louro J, Andrade CM, Sanchez AR, Pearse DD. Acute putrescine supplementation with Schwann cell implantation improves sensory and serotonergic axon growth and functional recovery in spinal cord injured rats. Neural Plast. 2015; 2015:186385.
Article24. Cheng Z, Bosco DB, Sun L, Chen X, Xu Y, Tai W, Didier R, Li J, Fan J, He X, Ren Y. Neural stem cell-conditioned medium suppresses inflammation and promotes spinal cord injury recovery. Cell Transplant. 2017; 26:469–482.
Article25. Zaminy A, Shokrgozar MA, Sadeghi Y, Norouzian M, Heidari MH, Piryaei A. Transplantation of schwann cells differentiated from adipose stem cells improves functional recovery in rat spinal cord injury. Arch Iran Med. 2013; 16:533–541.26. Edalat H, Hajebrahimi Z, Pirhajati V, Movahedin M, Tavallaei M, Soroush MR, Mowla SJ. Transplanting p75-suppressed bone marrow stromal cells promotes functional behavior in a rat model of spinal cord injury. Iran Biomed J. 2013; 17:140–145.27. Chen D, Zeng W, Fu Y, Gao M, Lv G. Bone marrow mesenchymal stem cells combined with minocycline improve spinal cord injury in a rat model. Int J Clin Exp Pathol. 2015; 8:11957–11969.28. Mehraein F, Golipoor Z. Transplant of very small embryoniclike stem cells to spinal cord injury in a rat model promotes movement recovery. Exp Clin Transplant. 2015; 13:256–261.29. Sarveazad A, Bakhtiari M, Babahajian A, Janzade A, Fallah A, Moradi F, Soleimani M, Younesi M, Goudarzi F, Joghataei MT. Comparison of human adipose-derived stem cells and chondroitinase ABC transplantation on locomotor recovery in the contusion model of spinal cord injury in rats. Iran J Basic Med Sci. 2014; 17:685–693.30. Neirinckx V, Agirman G, Coste C, Marquet A, Dion V, Rogister B, Franzen R, Wislet S. Adult bone marrow mesenchymal and neural crest stem cells are chemoattractive and accelerate motor recovery in a mouse model of spinal cord injury. Stem Cell Res Ther. 2015; 6:211.
Article31. Amemori T, Ruzicka J, Romanyuk N, Jhanwar-Uniyal M, Sykova E, Jendelova P. Comparison of intraspinal and intrathecal implantation of induced pluripotent stem cell-derived neural precursors for the treatment of spinal cord injury in rats. Stem Cell Res Ther. 2015; 6:257.
Article32. Nori S, Okada Y, Nishimura S, Sasaki T, Itakura G, Kobayashi Y, Renault-Mihara F, Shimizu A, Koya I, Yoshida R, Kudoh J, Koike M, Uchiyama Y, Ikeda E, Toyama Y, Nakamura M, Okano H. Long-term safety issues of iPSC-based cell therapy in a spinal cord injury model: oncogenic transformation with epithelialmesenchymal transition. Stem Cell Reports. 2015; 4:360–373.
Article33. Zaminy A, Ali Shokrgozar M, Sadeghi Y, Noroozian M, Hassan Heidari M, Piryaei A. Mesenchymal stem cells as an alternative for Schwann cells in rat spinal cord injury. Iran Biomed J. 2013; 17:113–122.34. Nicola FC, Rodrigues LP, Crestani T, Quintiliano K, Sanches EF, Willborn S, Aristimunha D, Boisserand L, Pranke P, Netto CA. Human dental pulp stem cells transplantation combined with treadmill training in rats after traumatic spinal cord injury. Braz J Med Biol Res. 2016; 49:e5319.
Article35. Du BL, Zeng X, Ma YH, Lai BQ, Wang JM, Ling EA, Wu JL, Zeng YS. Graft of the gelatin sponge scaffold containing genetically-modified neural stem cells promotes cell differentiation, axon regeneration, and functional recovery in rat with spinal cord transection. J Biomed Mater Res A. 2015; 103:1533–1545.
Article36. Abbaszadeh HA, Tiraihi T, Noori-Zadeh A, Delshad AR, Sadeghizade M, Taheri T. Human ciliary neurotrophic factor-overexpressing stable bone marrow stromal cells in the treatment of a rat model of traumatic spinal cord injury. Cytotherapy. 2015; 17:912–921.
Article37. Piltti KM, Funes GM, Avakian SN, Salibian AA, Huang KI, Carta K, Kamei N, Flanagan LA, Monuki ES, Uchida N, Cummings BJ, Anderson AJ. Increasing human neural stem cell transplantation dose alters oligodendroglial and neuronal differentiation after spinal cord injury. Stem Cell Reports. 2017; 8:1534–1548.
Article38. Abbaszadeh HA, Tiraihi T, Sadeghi Y, Delshad AR, Sadeghizadeh M, Taheri T, Noori-Zadeh A. Decrease in cavity size and oligodendrocyte cell death using neurosphere-derived oligodendrocyte-like cells in spinal cord contusion model. Iran Biomed J. 2018; 22:246–257.
Article39. Noori-Zadeh A, Mesbah-Namin SA, Bistoon-Beigloo S, Bakhtiyari S, Abbaszadeh HA, Darabi S, Rajabibazl M, Abdanipour A. Regulatory T cell number in multiple sclerosis patients: a meta-analysis. Mult Scler Relat Disord. 2016; 5:73–76.
Article40. Abdanipour A, Schluesener HJ, Tiraihi T, Noori-Zadeh A. Systemic administration of valproic acid stimulates overexpression of microtubule-associated protein 2 in the spinal cord injury model to promote neurite outgrowth. Neurol Res. 2015; 37:223–228.
Article41. Shams Nooraei M, Noori-Zadeh A, Darabi S, Rajaei F, Golmohammadi Z, Abbaszadeh HA. Low level of autophagy-related gene 10 (ATG10) expression in the 6-hydroxydopamine rat model of Parkinson's disease. Iran Biomed J. 2018; 22:15–21.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Concept and Future of the Management of Spinal Cord Injury: A Systematic Review
- Olig2-expressing Mesenchymal Stem Cells Enhance Functional Recovery after Contusive Spinal Cord Injury
- A Narrative Review of Advances in Neural Precursor Cell Transplantation Therapies for Spinal Cord Injury
- Effects of Transplantation of Human Embryonic Stem Cellson Functional Recovery in Spinal Cord Injured Rats
- The Time-course of Neurologic Recovery in Traumatic Spinal Cord Injury