Anat Cell Biol.
2019 Mar;52(1):48-56. 10.5115/acb.2019.52.1.48.
Nerve distribution in myocardium including the atrial and ventricular septa in late stage human fetuses
- Affiliations
-
- 1Department of Neurology, Wonkwang University School of Medicine and Hospital, Institute of Wonkwang Medical Science, Iksan, Korea.
- 2Department of Anatomy and Institute of Medical Sciences, Chonbuk National University Medical School, Jeonju, Korea. 407kk@hanmail.net
- 3Division of Internal Medicine, Asuka Hospital, Sapporo, Japan.
- 4Department of Anatomy, Akita University School of Medicine, Akita, Japan.
- 5Department of Anatomy and Human Embryology, Institute of Embryology, Faculty of Medicine, Complutense University, Madrid, Spain.
- KMID: 2442329
- DOI: http://doi.org/10.5115/acb.2019.52.1.48
Abstract
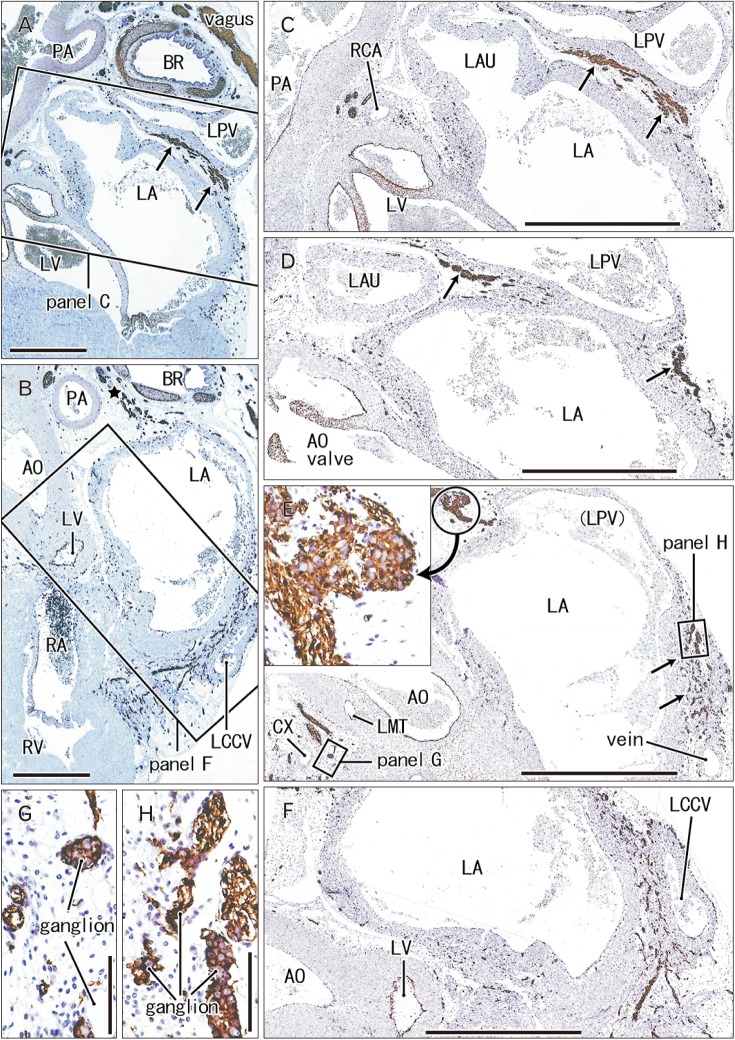
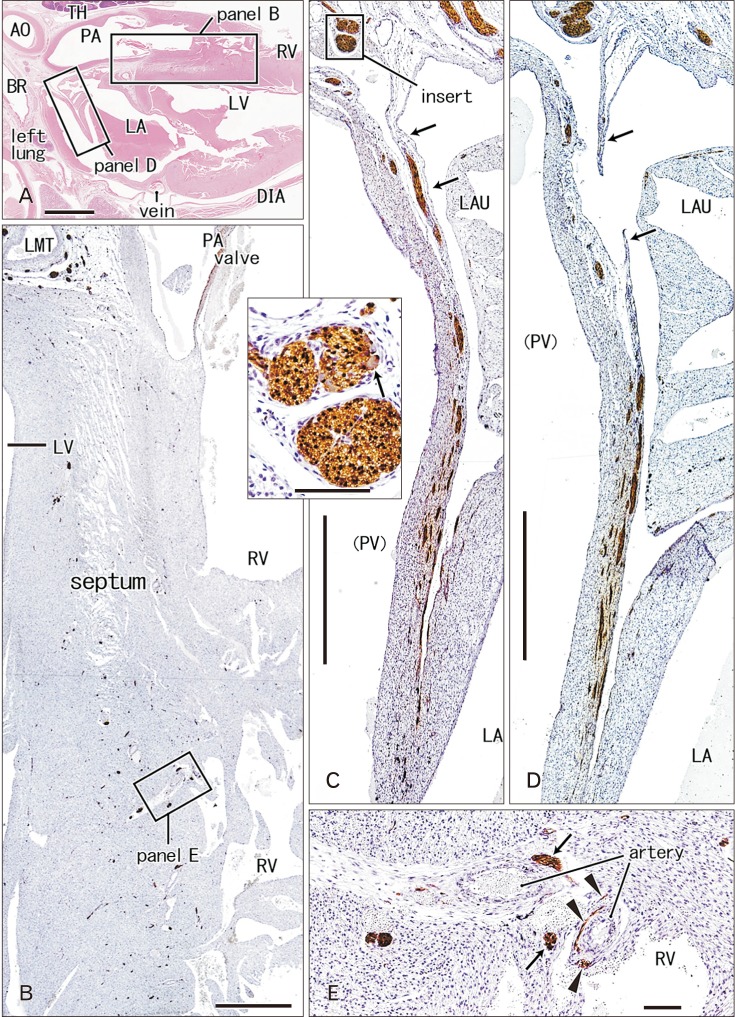
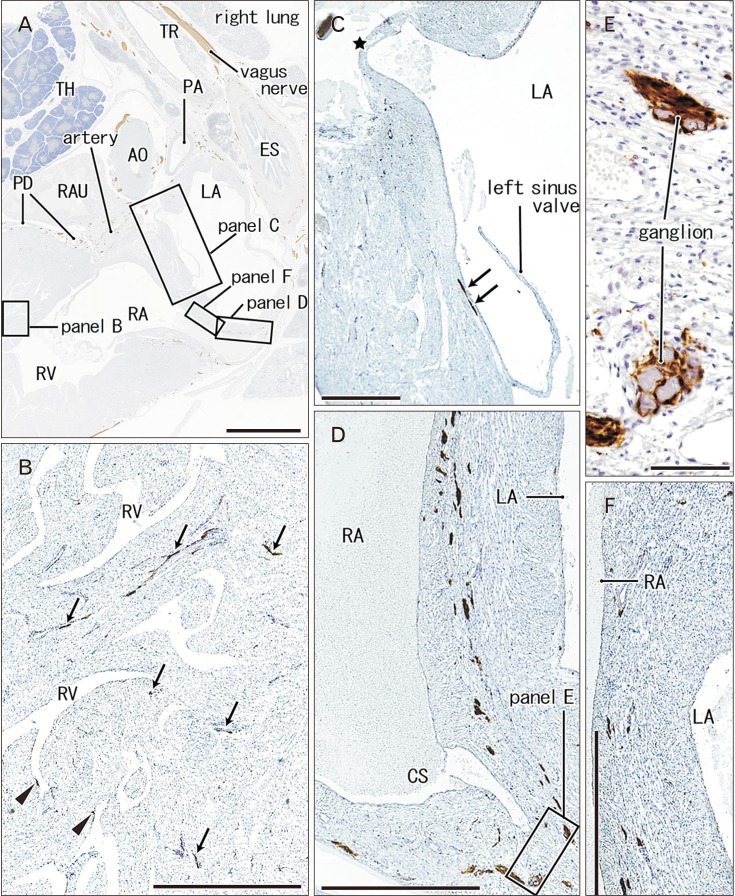
- Few information had been reported on deep intracardiac nerves in the myocardium of late human fetuses such as nerves at the atrial-pulmonary vein junction and in the atrial and ventricular septa. We examined histological sections of the heart obtained from 12 human fetuses at 25-33 weeks. A high density of intracardiac nerves was evident around the mitral valve annulus in contrast to few nerves around the tricuspid annulus. To the crux at the atrioventricular sulcus, the degenerating left common cardinal vein brought abundant nerve bundles coming from cardiac nerves descending along the anterior aspect of the pulmonary trunk. Likewise, nerve bundles in the left atrial nerve fold came from cardiac nerves between the ascending aorta and pulmonary artery. Conversely, another nerves from the venous pole to the atrium seemed to be much limited in number. Moreover, the primary atrial septum contained much fewer nerves than the secondary septum. Therefore, nerve density in the atrial wall varied considerably between sites. As ventricular muscles were degenerated from the luminal side for sculpturing of papillary muscles and trabeculae, deep nerves became exposed to the ventricular endothelium. Likewise, as pectineal muscles were sculptured, nerves were exposed in the atrial endothelium. Consequently, a myocardial assembly or sculpture seemed to be associated with degeneration and reconstruction of early-developed nerves. A failure in reconstruction during further expansion of the left atrium might be connected with an individual variation in anatomical substrates of atrial fibrillation.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Morphological and morphometric study of pulmonary vein anatomy in relation to cardiac invasive and electrophysiological procedures
Harshal Oza, Bhavik Doshi
Anat Cell Biol. 2023;56(4):428-434. doi: 10.5115/acb.23.141.
Reference
-
1. Pauza DH, Skripka V, Pauziene N, Stropus R. Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat Rec. 2000; 259:353–382. PMID: 10903529.2. Worobiew W. Plica nervina atrii sinistri. Z Anat Entwicklungsgesch. 1928; 86:509–516.3. Gardner E, O'Rahilly R. The nerve supply and conducting system of the human heart at the end of the embryonic period proper. J Anat. 1976; 121:571–587. PMID: 1018009.4. Tan AY, Li H, Wachsmann-Hogiu S, Chen LS, Chen PS, Fishbein MC. Autonomic innervation and segmental muscular disconnections at the human pulmonary vein-atrial junction: implications for catheter ablation of atrial-pulmonary vein junction. J Am Coll Cardiol. 2006; 48:132–143. PMID: 16814659.5. Nguyen BL, Fishbein MC, Chen LS, Chen PS, Masroor S. Histopathological substrate for chronic atrial fibrillation in humans. Heart Rhythm. 2009; 6:454–460. PMID: 19324302.6. Deneke T, Chaar H, de Groot JR, Wilde AA, Lawo T, Mundig J, Bösche L, Mügge A, Grewe PH. Shift in the pattern of autonomic atrial innervation in subjects with persistent atrial fibrillation. Heart Rhythm. 2011; 8:1357–1363. PMID: 21699826.7. Matsuyama TA, Tanaka H, Adachi T, Jiang Y, Ishibashi-Ueda H, Takamatsu T. Intrinsic left atrial histoanatomy as the basis for reentrant excitation causing atrial fibrillation/flutter in rats. Heart Rhythm. 2013; 10:1342–1348. PMID: 23680896.8. Marron K, Wharton J, Sheppard MN, Fagan D, Royston D, Kuhn DM, de Leval MR, Whitehead BF, Anderson RH, Polak JM. Distribution, morphology, and neurochemistry of endocardial and epicardial nerve terminal arborizations in the human heart. Circulation. 1995; 92:2343–2351. PMID: 7554220.9. Armour JA, Murphy DA, Yuan BX, Macdonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec. 1997; 247:289–298. PMID: 9026008.10. Singh S, Johnson PI, Javed A, Gray TS, Lonchyna VA, Wurster RD. Monoamine- and histamine-synthesizing enzymes and neurotransmitters within neurons of adult human cardiac ganglia. Circulation. 1999; 99:411–419. PMID: 9918529.11. Singh S, Gray T, Wurster RD. Nitric oxide and carbon monoxide synthesizing enzymes and soluble guanylyl cyclase within neurons of adult human cardiac ganglia. Auton Neurosci. 2009; 145:93–98. PMID: 19106038.12. Hoover DB, Isaacs ER, Jacques F, Hoard JL, Pagé P, Armour JA. Localization of multiple neurotransmitters in surgically derived specimens of human atrial ganglia. Neuroscience. 2009; 164:1170–1179. PMID: 19747529.13. Taguchi K, Tsukamoto T, Murakami G. Anatomical studies of the autonomic nervous system in the human pelvis by the whole-mount staining method: left-right communicating nerves between bilateral pelvic plexuses. J Urol. 1999; 161:320–325. PMID: 10037431.14. Orts Llorca F, Domenech Mateu JM, Puerta Fonolla J. Innervation of the sinu-atrial node and neighbouring regions in two human embryos. J Anat. 1979; 128:365–375. PMID: 438095.15. Hachisuka H, Mori O, Sakamoto F, Sasai Y, Nomura H. Immunohistological demonstration of S-100 protein in the cutaneous nervous system. Anat Rec. 1984; 210:639–646. PMID: 6524701.16. Captur G, Wilson R, Bennett MF, Luxán G, Nasis A, de la Pompa JL, Moon JC, Mohun TJ. Morphogenesis of myocardial trabeculae in the mouse embryo. J Anat. 2016; 229:314–325. PMID: 27020702.17. Cheng G, Wessels A, Gourdie RG, Thompson RP. Spatiotemporal and tissue specific distribution of apoptosis in the developing chick heart. Dev Dyn. 2002; 223:119–133. PMID: 11803575.18. Zhao Z, Rivkees SA. Programmed cell death in the developing heart: regulation by BMP4 and FGF2. Dev Dyn. 2000; 217:388–400. PMID: 10767083.19. Sedmera D, Pexieder T, Vuillemin M, Thompson RP, Anderson RH. Developmental patterning of the myocardium. Anat Rec. 2000; 258:319–337. PMID: 10737851.20. Oosthoek PW, Wenink AC, Wisse LJ, Gittenberger-de Groot AC. Development of the papillary muscles of the mitral valve: morphogenetic background of parachute-like asymmetric mitral valves and other mitral valve anomalies. J Thorac Cardiovasc Surg. 1998; 116:36–46. PMID: 9671895.21. Singh S, Johnson PI, Lee RE, Orfei E, Lonchyna VA, Sullivan HJ, Montoya A, Tran H, Wehrmacher WH, Wurster RD. Topography of cardiac ganglia in the adult human heart. J Thorac Cardiovasc Surg. 1996; 112:943–953. PMID: 8873720.22. Pasque M, Williams WG, Coles JG, Trusler GA, Freedom RM. Tricuspid valve replacement in children. Ann Thorac Surg. 1987; 44:164–168. PMID: 3619539.23. Ross FJ, Latham GJ, Richards M, Geiduschek J, Thompson D, Joffe D. Perioperative and anesthetic considerations in Ebstein's anomaly. Semin Cardiothorac Vasc Anesth. 2016; 20:82–92. PMID: 26472205.24. Kim JH, Cho KH, Jin ZW, Murakami G, Abe H, Chai OH. Ganglion cardiacum or juxtaductal body of human fetuses. Anat Cell Biol. 2018; 51:266–273. PMID: 30637161.25. Zhao QY, Huang H, Zhang SD, Tang YH, Wang X, Zhang YG, Salim M, Okello E, Deng HP, Yu SB, Huang CX. Atrial autonomic innervation remodelling and atrial fibrillation inducibility after epicardial ganglionic plexi ablation. Europace. 2010; 12:805–810. PMID: 20353962.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Left Ventricular Noncompaction Accompanying Fasciculo-Ventricular Accessory Pathway and Atrial Flutter
- Studies on the Development of Lung and Distribution of Elastic and Reticular Fibers during Fetal Period Proper
- Stroke in a Young Individual with Left Ventricular Noncompaction and Left Atrium Standstill
- A case of spongy myocardium initially manifested by ventricular tachycardia in adult
- Atrial Fibrillation in a Patient with Left Ventricular Hypertrophy after Induction of General Anesthesia: A case report