Investig Magn Reson Imaging.
2019 Mar;23(1):26-33. 10.13104/imri.2019.23.1.26.
Mini-Review of Studies Reporting the Repeatability and Reproducibility of Diffusion Tensor Imaging
- Affiliations
-
- 1Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University, Daegu, Korea. strokerehab@hanmail.net
- KMID: 2442230
- DOI: http://doi.org/10.13104/imri.2019.23.1.26
Abstract
- PURPOSE
Diffusion tensor imaging (DTI) data must be analyzed by an analyzer after data processing. Hence, the analyzed data of DTI might depend on the analyzer, making it a major limitation. This paper reviewed previous DTI studies reporting the repeatability and reproducibility of data from the corticospinal tract (CST), one of the most actively researched neural tracts on this topic.
MATERIALS AND METHODS
Relevant studies published between January 1990 and December 2018 were identified by searching PubMed, Google Scholar, and MEDLINE electronic databases using the following keywords: DTI, diffusion tensor tractography, reliability, repeatability, reproducibility, and CST. As a result, 15 studies were selected.
RESULTS
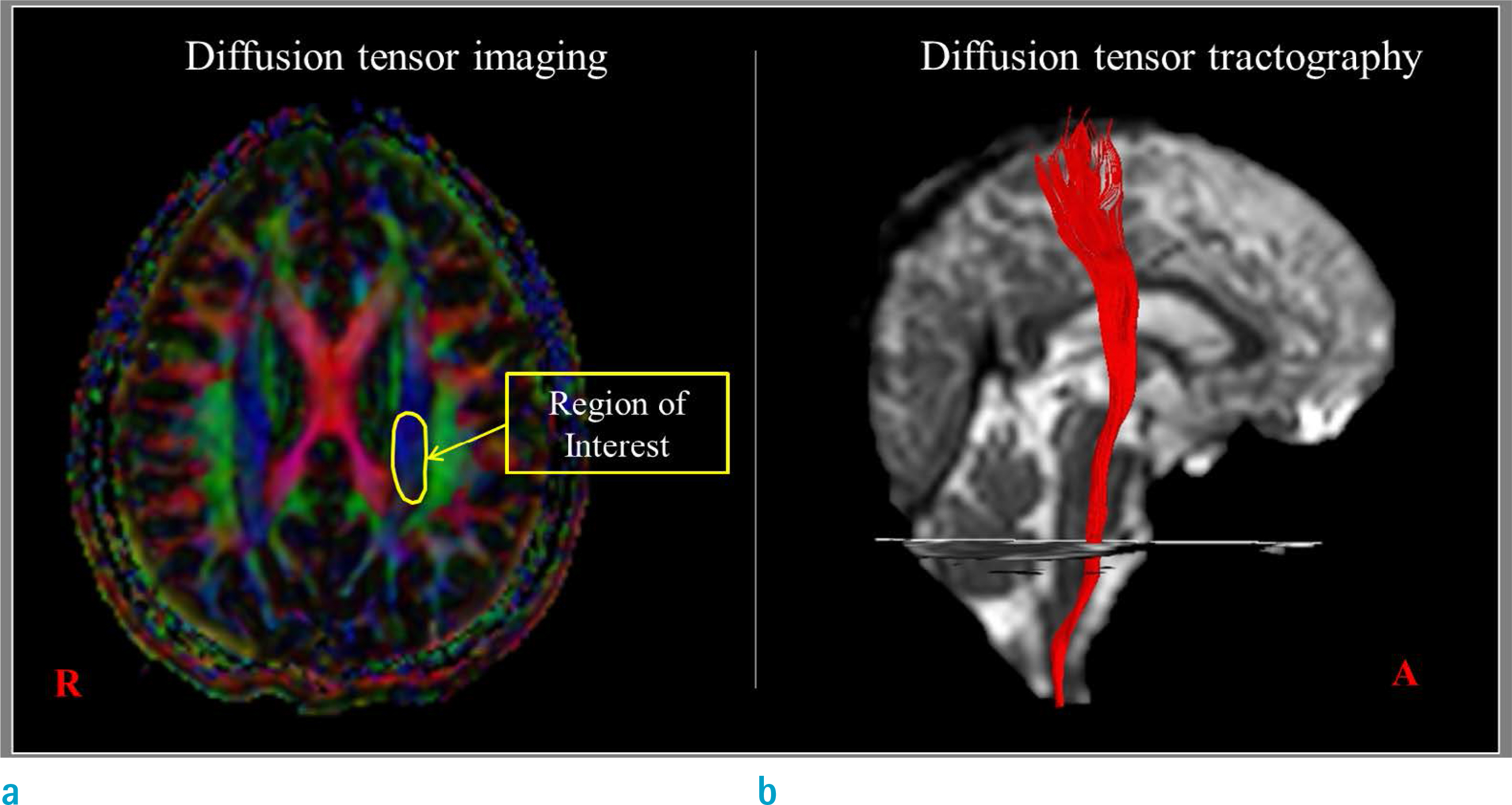
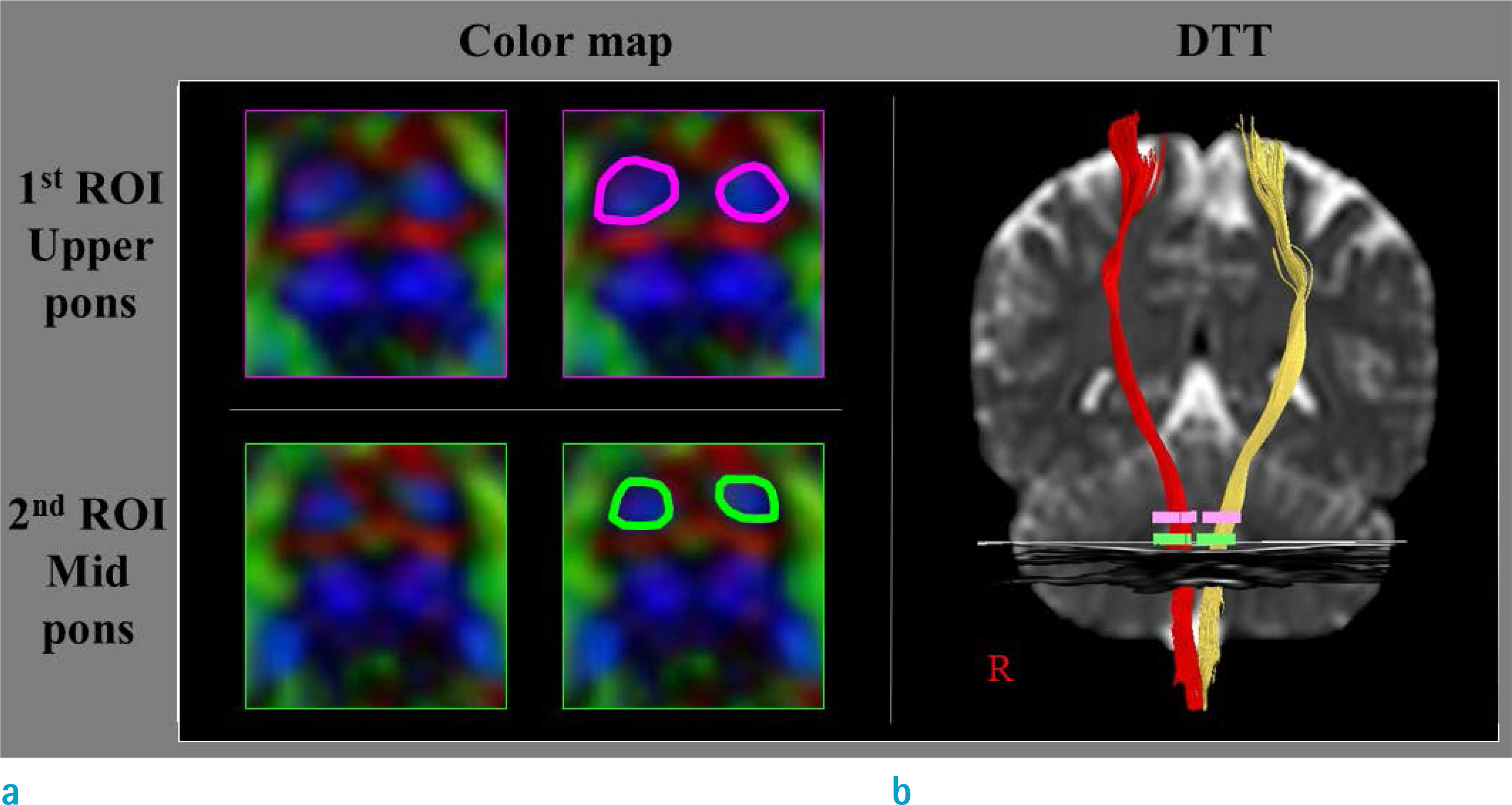
Measurements of the CSTs using region of interest methods on 2-dimensional DTI images generally showed excellent repeatability and reproducibility of more than 0.8 but high variability (0.29 to 1.00) between studies. In contrast, measurements of the CST using the 3-dimensional DTT method not only revealed excellent repeatability and reproducibility of more than 0.9 but also low variability (repeatability, 0.88 to 1.00; reproducibility, 0.82 to 0.99) between studies.
CONCLUSION
Both 2-dimensional DTI and 3-dimensional DTT methods appeared to be reliable for measuring the CST but the 3-dimensional DTT method appeared to be more reliable.
Keyword
Figure
Reference
-
References
1. Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994; 66:259–267.
Article2. Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999; 45:265–269.
Article3. Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn Reson Med. 2000; 44:625–632.
Article4. Wang JY, Bakhadirov K, Devous MD Sr, et al. Diffusion tensor tractography of traumatic diffuse axonal injury. Arch Neurol. 2008; 65:619–626.
Article5. Shenton ME, Hamoda HM, Schneiderman JS, et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 2012; 6:137–192.
Article6. Jang SH. Traumatic axonal injury in mild traumatic brain injury. In: Gorbunoy N, ed. Traumatic brain injury. 1st ed. London: InTech. 2018. 137–154.7. Yu FF, Chiang FL, Stephens N, et al. Characterization of normal-appearing white matter in multiple sclerosis using quantitative susceptibility mapping in conjunction with diffusion tensor imaging. Neuroradiology. 2019; 61:71–79.
Article8. Lee SK, Kim DI, Kim J, et al. Diffusion-tensor MR imaging and fiber tractography: a new method of describing aberrant fiber connections in developmental CNS anomalies. Radiographics. 2005; 25:53–65. discussion 66–58.
Article9. Yamada K, Sakai K, Akazawa K, Yuen S, Nishimura T. MR tractography: a review of its clinical applications. Magn Reson Med Sci. 2009; 8:165–174.
Article10. Stieltjes B, Kaufmann WE, van Zijl PC, et al. Diffusion tensor imaging and axonal tracking in the human brainstem. Neuroimage. 2001; 14:723–735.
Article11. Bonekamp D, Nagae LM, Degaonkar M, et al. Diffusion tensor imaging in children and adolescents: reproducibility, hemispheric, and age-related differences. Neuroimage. 2007; 34:733–742.
Article12. Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007; 36:630–644.
Article13. Danielian LE, Iwata NK, Thomasson DM, Floeter MK. Reliability of fiber tracking measurements in diffusion tensor imaging for longitudinal study. Neuroimage. 2010; 49:1572–1580.
Article14. Borich MR, Wadden KP, Boyd LA. Establishing the reproducibility of two approaches to quantify white matter tract integrity in stroke. Neuroimage. 2012; 59:2393–2400.
Article15. Hakulinen U, Brander A, Ryymin P, et al. Repeatability and variation of region-of-interest methods using quantitative diffusion tensor MR imaging of the brain. BMC Med Imaging. 2012; 12:30.
Article16. Lee AY, Shin DG, Park JS, et al. Neural tracts injuries in patients with hypoxic ischemic brain injury: diffusion tensor imaging study. Neurosci Lett. 2012; 528:16–21.
Article17. Jang SH, Chang CH, Lee J, Kim CS, Seo JP, Yeo SS. Functional role of the corticoreticular pathway in chronic stroke patients. Stroke. 2013; 44:1099–1104.
Article18. Kristo G, Leemans A, de Gelder B, Raemaekers M, Rutten GJ, Ramsey N. Reliability of the corticospinal tract and arcuate fasciculus reconstructed with DTI-based tractography: implications for clinical practice. Eur Radiol. 2013; 23:28–36.
Article19. Kwon HG, Son SM, Jang SH. Development of the transcallosal motor fiber from the corticospinal tract in the human brain: diffusion tensor imaging study. Front Hum Neurosci. 2014; 8:153.
Article20. Paldino MJ, Hedges K, Rodrigues KM, Barboriak DP. Repeatability of quantitative metrics derived from MR diffusion tractography in paediatric patients with epilepsy. Br J Radiol. 2014; 87:20140095.
Article21. Rijken BF, Leemans A, Lucas Y, van Montfort K, Mathijssen IM, Lequin MH. Diffusion tensor imaging and fiber tractography in children with craniosynostosis syndromes. AJNR Am J Neuroradiol. 2015; 36:1558–1564.
Article22. Acheson A, Wijtenburg SA, Rowland LM, et al. Reproducibility of tract-based white matter microstructural measures using the ENIGMA-DTI protocol. Brain Behav. 2017; 7:e00615.
Article23. Ius T, Turella L, Pauletto G, et al. Quantitative diffusion tensor imaging analysis of low-grade gliomas: from preclinical application to patient care. World Neurosurg. 2017; 97:333–343.
Article24. Rosenstock T, Giampiccolo D, Schneider H, et al. Specific DTI seeding and diffusivity-analysis improve the quality and prognostic value of TMS-based deterministic DTI of the pyramidal tract. Neuroimage Clin. 2017; 16:276–285.
Article25. Kunimatsu A, Aoki S, Masutani Y, et al. The optimal trackability threshold of fractional anisotropy for diffusion tensor tractography of the corticospinal tract. Magn Reson Med Sci. 2004; 3:11–17.
Article26. Seo JP, Chang PH, Jang SH. Anatomical location of the corticospinal tract according to somatotopies in the centrum semiovale. Neurosci Lett. 2012; 523:111–114.
Article27. Kwon HG, Yang JH, Park JB, Kim MH, Choi SH, Yang DS. Anatomical location and somatotopic organization of the corticospinal tract in the corona radiata of the normal human brain: a diffusion tensor tractography study. Neuroreport. 2014; 25:710–714.28. Wang JY, Abdi H, Bakhadirov K, Diaz-Arrastia R, Devous MD Sr. A comprehensive reliability assessment of quantitative diffusion tensor tractography. Neuroimage. 2012; 60:1127–1138.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Principle and Experiments in Diffusion Tensor Imaging
- Diffusion Tensor Imaging: Exploring the Motor Networks and Clinical Applications
- Multi-slice Multi-echo Pulsed-gradient Spin-echo (MePGSE) Sequence for Diffusion Tensor Imaging MRI: A Preliminary Result
- Central Pain Due to Traumatic Axonal Injury of the Spinothalamic Tract in Patients with Mild Traumatic Brain Injury
- Brain Diffusion Tensor MR Imaging