J Rheum Dis.
2019 Apr;26(2):118-123. 10.4078/jrd.2019.26.2.118.
Comparison of Efficacy and Safety of Febuxostat in Gout Patients with Chronic Kidney Disease Stage 3 and Stage 4/5
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, Pusan National University Yangsan Hospital, Yangsan, Korea. thalsdnrso@naver.com
- 2Division of Rheumatology, Department of Internal Medicine, Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea.
- 3Division of Rheumatology, Department of Internal Medicine, Pusan National University Hospital, Busan, Korea.
- KMID: 2442022
- DOI: http://doi.org/10.4078/jrd.2019.26.2.118
Abstract
OBJECTIVE
To compare efficacy and safety of febuxostat in gouty patients with chronic kidney disease (CKD) stage 3 and stage 4/5.
METHODS
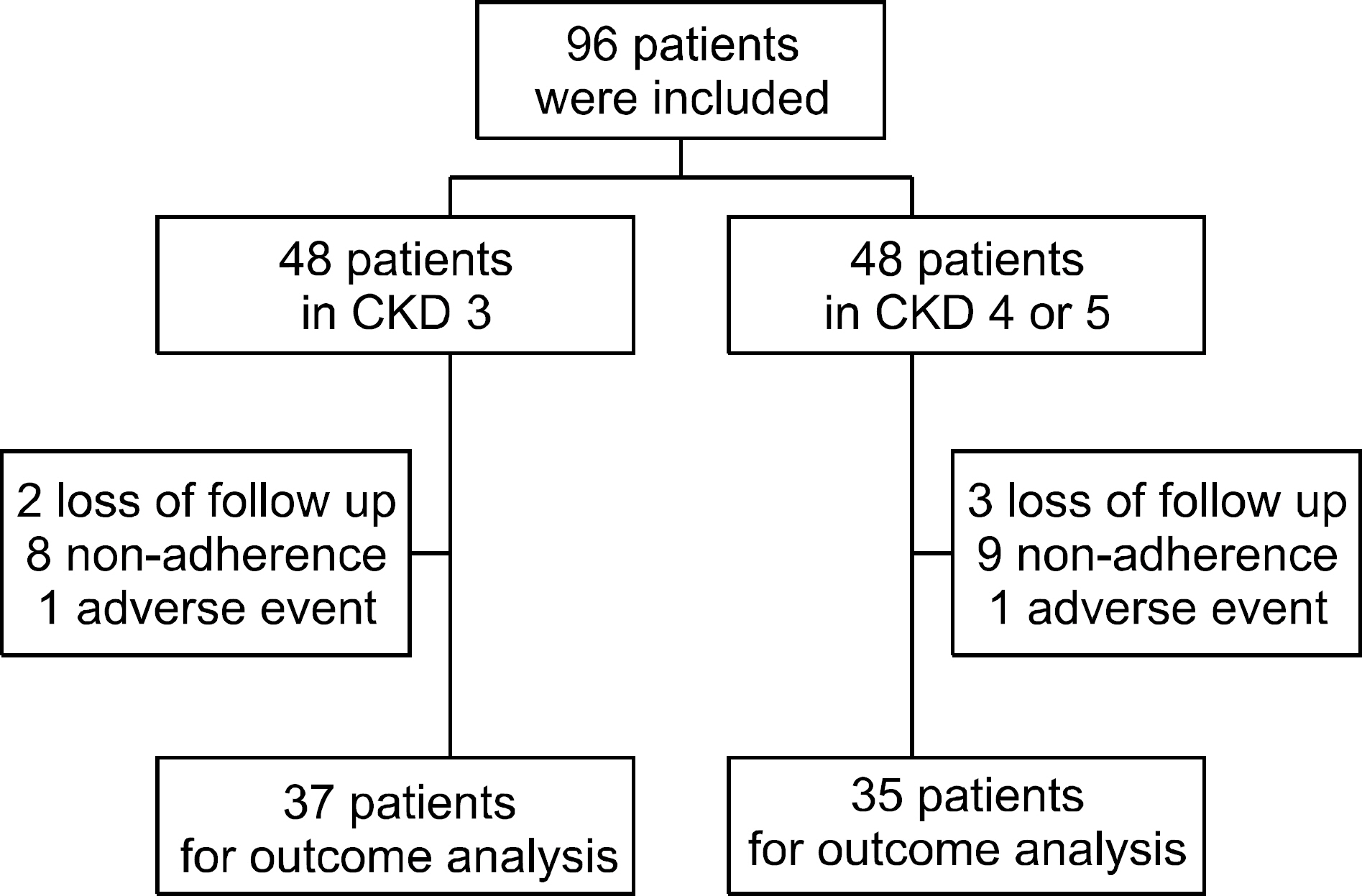
Age and sex matched patients with CKD stage 3 and stage 4/5 who were diagnosed with gout were included. The dose of febuxostat was increased according to serum uric acid (sUA) level. Adherence, the number of gout attack, the change of sUA, the change of estimated glomerular filtration rate (eGFR) and adverse events (AEs) were evaluated for 12 months.
RESULTS
There were no significant differences in the baseline variables between CKD stage 3 and CKD stage 4/5. Disease duration was longer and baseline sUA was higher in the CKD stage 4/5. There were no significant differences in the mean sUA at the last follow-up, the number of patients who reached the sUA target of 6 mg/dL and the number of gout attack between the groups. There were no significant differences in the change of eGFR and decrease of eGFR between the groups. There were 2 cases of AEs. One patient in CKD stage 3 had maculopapular rash and one patient in CKD stage 4/5 had dizziness. The AEs were subsided after febuxostat was stopped.
CONCLUSION
Febuxostat was efficacious and well tolerated in gout patients with CKD stage 4/5.
Keyword
MeSH Terms
Figure
Reference
-
1. Schlesinger N. Difficult-to-treat gouty arthritis: a disease warranting better management. Drugs. 2011; 71:1413–39.2. Fuldeore MJ, Riedel AA, Zarotsky V, Pandya BJ, Dabbous O, Krishnan E. Chronic kidney disease in gout in a managed care setting. BMC Nephrol. 2011; 12:36.
Article3. McClory J, Said N. Gout in women. Med Health R I. 2009; 92:363–4. 368.4. Shoji A, Yamanaka H, Kamatani N. A retrospective study of the relationship between serum urate level and recurrent attacks of gouty arthritis: evidence for reduction of recurrent gouty arthritis with antihyperuricemic therapy. Arthritis Rheum. 2004; 51:321–5.
Article5. Richette P, Doherty M, Pascual E, Barskova V, Becce F, Castañeda-Sanabria J, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2017; 76:29–42.
Article6. Richette P, Frazier A, Bardin T. Pharmacokinetics considerations for gout treatments. Expert Opin Drug Metab Toxicol. 2014; 10:949–57.
Article7. Dalbeth N, Kumar S, Stamp L, Gow P. Dose adjustment of allopurinol according to creatinine clearance does not provide adequate control of hyperuricemia in patients with gout. J Rheumatol. 2006; 33:1646–50.8. Mukoyoshi M, Nishimura S, Hoshide S, Umeda S, Kanou M, Taniguchi K, et al. In vitro drug-drug interaction studies with Febuxostat, a novel non-purine selective inhibitor of xanthine oxidase: plasma protein binding, identification of metabolic enzymes and cytochrome P450 inhibition. Xenobiotica. 2008; 38:496–510.9. Hira D, Chisaki Y, Noda S, Araki H, Uzu T, Maegawa H, et al. Population pharmacokinetics and therapeutic efficacy of Febuxostat in patients with severe renal impairment. Pharmacology. 2015; 96:90–8.
Article10. Schumacher HR Jr, Becker MA, Wortmann RL, Macdonald PA, Hunt B, Streit J, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum. 2008; 59:1540–8.
Article11. Juge PA, Truchetet ME, Pillebout E, Ottaviani S, Vigneau C, Loustau C, et al. Efficacy and safety of febuxostat in 73 gouty patients with stage 4/5 chronic kidney disease: a retrospective study of 10 centers. Joint Bone Spine. 2017; 84:595–8.
Article12. Quilis N, Andrés M, Gil S, Ranieri L, Vela P, Pascual E. Febuxostat for patients with gout and severe chronic kidney disease: which is the appropriate dosage? Comment on the article by Saag et al. Arthritis Rheumatol. 2016; 68:2563–4.
Article13. Lim DH, Oh JS, Ahn SM, Hong S, Kim YG, Lee CK, et al. Febuxostat in hyperuricemic patients with advanced CKD. Am J Kidney Dis. 2016; 68:819–21.
Article14. Sakai Y, Otsuka T, Ohno D, Murasawa T, Sato N, Tsuruoka S. Febuxostat for treating allopurinol-resistant hyperuricemia in patients with chronic kidney disease. Ren Fail. 2014; 36:225–31.
Article15. Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yü TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977; 20:895–900.
Article16. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999; 130:461–70.17. Saag KG, Whelton A, Becker MA, MacDonald P, Hunt B, Gunawardhana L. Impact of Febuxostat on renal function in gout patients with moderate-to-severe renal impairment. Arthritis Rheumatol. 2016; 68:2035–43.
Article18. Sircar D, Chatterjee S, Waikhom R, Golay V, Raychaudhury A, Chatterjee S, et al. Efficacy of Febuxostat for slowing the GFR decline in patients with CKD and asymptomatic hyperuricemia: a 6-month, double-blind, randomized, place-bo-controlled trial. Am J Kidney Dis. 2015; 66:945–50.
Article19. Lee S, So MW. Adherence with urate-lowering therapies among male patients with gout in a routine clinical setting. Mod Rheumatol. 2016; 26:950–5.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Renal safety and urate-lowering efficacy of febuxostat in gout patients with stage 4–5 chronic kidney disease not yet on dialysis
- The Efficacy and Safety of Febuxostat in Korean Patients with Gout
- Comparative Efficacy and Safety of Febuxostat and Allopurinol in the Treatment of Hyperuricemia: A Bayesian Network Meta-analysis
- Febuxostat for the Treatment of Chronic Tophaceous Gout in a Patient on Continuous Ambulatory Peritoneal Dialysis
- What is the Best Choice for Urate-lowering Therapy for Korean?