Neonatal Med.
2019 Feb;26(1):41-47. 10.5385/nm.2019.26.1.41.
Analysis of the Influencing Factors of 17-Hydroxyprogesterone Level and the Correlation between 17-Hydroxyprogesterone Level and the Clinical Parameters Related to Adrenal Cortical Function in Very-Low-Birth-Weight Infants
- Affiliations
-
- 1Department of Pediatrics, Ajou University Hospital, Ajou University School of Medicine, Suwon, Korea. neopedlee@ajou.ac.kr
- 2Department of Medical Genetics, Ajou University Hospital, Ajou University School of Medicine, Suwon, Korea.
- 3Department of Radiation Oncology, Ajou University Hospital, Ajou University School of Medicine, Suwon, Korea.
- 4Department of Biomedical Informatics, Ajou University Hospital, Ajou University School of Medicine, Suwon, Korea.
- KMID: 2440699
- DOI: http://doi.org/10.5385/nm.2019.26.1.41
Abstract
- PURPOSE
17-Hydroxyprogesterone (17-OHP) screening results are difficult to interpret owing to the many influencing factors, and confirming the test results takes time. In this study, we examined the factors that affected the 17-OHP level in premature infants. We also evaluated the correlation between 17-OHP level and the clinical parameters related to adrenal cortical function.
METHODS
From January 2012 to April 2017, 358 very-low-birth-weight infants (VLBWI) born with birth weights of < 1,500 g were included in the study. Their 17-OHP levels were measured in the neonatal screening test after birth and analyzed by considering various factors that may have influenced the values.
RESULTS
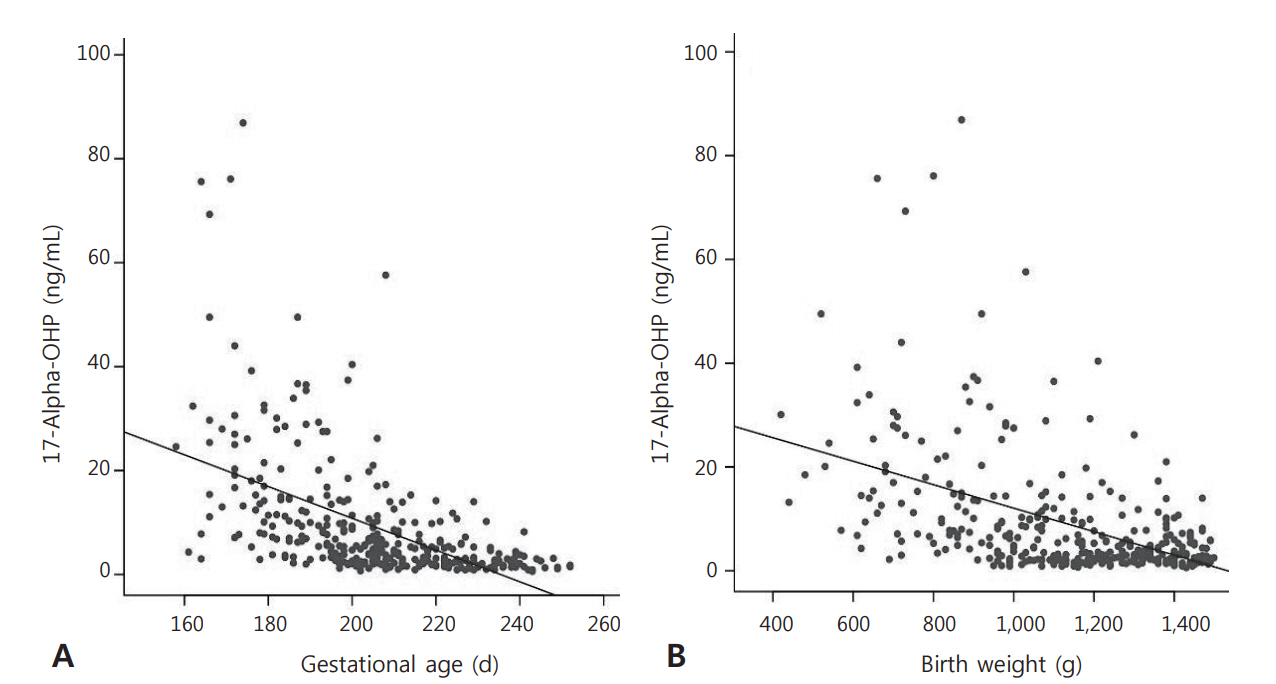
The 17-OHP levels negatively correlated with gestational age and birth weight. The values of the parameters that affected the 17-OHP levels were significantly higher in the infants with respiratory distress syndrome (RDS). In relation to the clinical parameters, blood pressure measured within 24 hours, 72 hours, and 1 week after birth negatively correlated with the 17-OHP level. Serum sodium and 17-OHP levels 24 hours after birth were found to be positively correlated. Urine outputs in 1 and 3 days after birth showed significant positive correlations with the 17-OHP level.
CONCLUSION
The 17-OHP levels of the VLBWIs were higher when gestational age and birth weight were lower, and were influenced by RDS in the VLBWI. In addition, hypotension and urine output values may be useful in the neonatal intensive care unit as a predictor of early adrenal insufficiency.
Keyword
MeSH Terms
-
17-alpha-Hydroxyprogesterone*
Adrenal Hyperplasia, Congenital
Adrenal Insufficiency
Birth Weight
Blood Pressure
Gestational Age
Humans
Hypotension
Infant
Infant, Newborn
Infant, Premature
Infant, Very Low Birth Weight*
Intensive Care, Neonatal
Mass Screening
Neonatal Screening
Parturition
Sodium
17-alpha-Hydroxyprogesterone
Sodium
Figure
Reference
-
1. Hughes IA. Congenital adrenal hyperplasia: a continuum of disorders. Lancet. 1998; 352:752–4.2. Speiser PW, White PC. Congenital adrenal hyperplasia. N Engl J Med. 2003; 349:776–88.3. Barra CB, Silva IN, Pezzuti IL, Januario JN. Neonatal screening for congenital adrenal hyperplasia. Rev Assoc Med Bras (1992). 2012; 58:459–64.4. Riepe FG, Sippell WG. Recent advances in diagnosis, treatment, and outcome of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Rev Endocr Metab Disord. 2007; 8:349–63.5. White PC, Speiser PW. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev. 2000; 21:245–91.6. Sharma R, Seth A. Congenital adrenal hyperplasia: issues in diagnosis and treatment in children. Indian J Pediatr. 2014; 81:178–85.7. Pang S, Hotchkiss J, Drash AL, Levine LS, New MI. Microfilter paper method for 17 alpha-hydroxyprogesterone radioimmunoassay: its application for rapid screening for congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1977; 45:1003–8.8. Gruneiro-Papendieck L, Prieto L, Chiesa A, Bengolea S, Bossi G, Bergada C. Neonatal screening program for congenital adrenal hyperplasia: adjustments to the recall protocol. Horm Res. 2001; 55:271–7.9. Honour JW, Torresani T. Evaluation of neonatal screening for congenital adrenal hyperplasia. Horm Res. 2001; 55:206–11.10. Ballerini MG, Chiesa A, Scaglia P, Gruneiro-Papendieck L, Heinrich JJ, Ropelato MG. 17Alpha-hydroxyprogesterone and cortisol serum levels in neonates and young children: influence of age, gestational age, gender and methodological procedures. J Pediatr Endocrinol Metab. 2010; 23:121–32.11. Masumoto K, Kusuda S, Aoyagi H, Tamura Y, Obonai T, Yamasaki C, et al. Comparison of serum cortisol concentrations in preterm infants with or without late-onset circulatory collapse due to adrenal insufficiency of prematurity. Pediatr Res. 2008; 63:686–90.12. Steigert M, Schoenle EJ, Biason-Lauber A, Torresani T. High reliability of neonatal screening for congenital adrenal hyperplasia in Switzerland. J Clin Endocrinol Metab. 2002; 87:4106–10.13. Cavarzere P, Camilot M, Teofoli F, Tato L. Neonatal screening for congenital adrenal hyperplasia in North-Eastern Italy: a report three years into the program. Horm Res. 2005; 63:180–6.14. van der Kamp HJ, Oudshoorn CG, Elvers BH, van Baarle M, Otten BJ, Wit JM, et al. Cutoff levels of 17-alpha-hydroxyprogesterone in neonatal screening for congenital adrenal hyperplasia should be based on gestational age rather than on birth weight. J Clin Endocrinol Metab. 2005; 90:3904–7.15. Allen DB, Hoffman GL, Fitzpatrick P, Laessig R, Maby S, Slyper A. Improved precision of newborn screening for congenital adrenal hyperplasia using weight-adjusted criteria for 17-hydroxyprogesterone levels. J Pediatr. 1997; 130:128–33.16. Olgemoller B, Roscher AA, Liebl B, Fingerhut R. Screening for congenital adrenal hyperplasia: adjustment of 17-hydroxyprogesterone cut-off values to both age and birth weight markedly improves the predictive value. J Clin Endocrinol Metab. 2003; 88:5790–4.17. Kunze M, Hart JE, Lynch AM, Gibbs RS. Intrapartum management of premature rupture of membranes: effect on cesarean delivery rate. Obstet Gynecol. 2011; 118:1247–54.18. Ersch J, Beinder E, Stallmach T, Bucher HU, Torresani T. 17-Hydroxyprogesterone in premature infants as a marker of intrauterine stress. J Perinat Med. 2008; 36:157–60.19. Paul DA, Leef KH, Stefano JL, Bartoshesky L. Factors influencing levels of 17-hydroxyprogesterone in very low birth weight infants and the relationship to death and IVH. J Perinatol. 2004; 24:252–6.20. Chung HR, Shin CH, Yang SW, Yun KA, Lee YA, Park SE, et al. Interpretation of screening for congenital adrenal hyperplasia in preterm infants. Korean J Pediatr. 2008; 51:616–21.21. Sweet D, Bevilacqua G, Carnielli V, Greisen G, Plavka R, Saugstad OD, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome. J Perinat Med. 2007; 35:175–86.22. R Core Team. R: a language and environment for the statistical computing [Internet]. Vienna: R Foundation for Statistical Computing;2019. [cited 2019 Jan 4]. Available from: https://cran.r-project.org/mirrors.html.23. Cavarzere P, Samara-Boustani D, Flechtner I, Dechaux M, Elie C, Tardy V, et al. Transient hyper-17-hydroxyprogesteronemia: a clinical subgroup of patients diagnosed at neonatal screening for congenital adrenal hyperplasia. Eur J Endocrinol. 2009; 161:285–92.24. Choi YS, Lee BS, Kim KS, Kim EA. Study of 17-alpha-hydroxy progesterone in preterm infants. J Korean Soc Neonatol. 2012; 19:77–83.25. Ryckman KK, Cook DE, Berberich SL, Shchelochkov OA, Berends SK, Busch T, et al. Replication of clinical associations with 17-hydroxyprogesterone in preterm newborns. J Pediatr Endocrinol Metab. 2012; 25:301–5.26. Chennuri VS, Mithbawkar SM, Mokal RA, Desai MP. Serum 17 alpha hydroxyprogesterone in normal full term and preterm vs sick preterm and full term newborns in a tertiary hospital. Indian J Pediatr. 2013; 80:21–5.27. Anandi VS, Shaila B. Evaluation of factors associated with elevated newborn 17-hydroxyprogesterone levels. J Pediatr Endocrinol Metab. 2017; 30:677–81.28. King JL, Naber JM, Hopkin RJ, Repaske DR, Bailey L, Leslie ND. Antenatal corticosteroids and newborn screening for congenital adrenal hyperplasia. Arch Pediatr Adolesc Med. 2001; 155:1038–42.29. Gatelais F, Berthelot J, Beringue F, Descamps P, Bonneau D, Limal JM, et al. Effect of single and multiple courses of prenatal corticosteroids on 17-hydroxyprogesterone levels: implication for neonatal screening of congenital adrenal hyperplasia. Pediatr Res. 2004; 56:701–5.30. Travers S, Martinerie L, Boileau P, Lombes M, Pussard E. Alterations of adrenal steroidomic profiles in preterm infants at birth. Arch Dis Child Fetal Neonatal Ed. 2018; 103:F143–51.31. Ng PC, Lam CW, Fok TF, Lee CH, Ma KC, Chan IH, et al. Refractory hypotension in preterm infants with adrenocortical insufficiency. Arch Dis Child Fetal Neonatal Ed. 2001; 84:F122–4.32. Ng PC, Lee CH, Lam CW, Ma KC, Fok TF, Chan IH, et al. Transient adrenocortical insufficiency of prematurity and systemic hypotension in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 2004; 89:F119–26.33. Berry J, Betts P, Wood PJ. The interpretation of bloodspot 17 alpha-hydroxyprogesterone levels in term and pre-term neonates. Ann Clin Biochem. 1986; 23(Pt 5):546–51.34. Ohkubo S, Shimozawa K, Matsumoto M, Kitagawa T. Analysis of blood spot 17 alpha-hydroxyprogesterone concentration in premature infants: proposal for cut-off limits in screening for congenital adrenal hyperplasia. Acta Paediatr Jpn. 1992; 34:126–33.35. Hingre RV, Gross SJ, Hingre KS, Mayes DM, Richman RA. Adrenal steroidogenesis in very low birth weight preterm infants. J Clin Endocrinol Metab. 1994; 78:266–70.36. Huysman MW, Hokken-Koelega AC, De Ridder MA, Sauer PJ. Adrenal function in sick very preterm infants. Pediatr Res. 2000; 48:629–33.37. Riepe FG, Mahler P, Sippell WG, Partsch CJ. Longitudinal study of plasma pregnenolone and 17-hydroxypregnenolone in fullterm and preterm neonates at birth and during the early neonatal period. J Clin Endocrinol Metab. 2002; 87:4301–6.38. Krasowski MD, Drees D, Morris CS, Maakestad J, Blau JL, Ekins S. Cross-reactivity of steroid hormone immunoassays: clinical significance and two-dimensional molecular similarity prediction. BMC Clin Pathol. 2014; 14:33.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cutoff Value of 17-Hydroxyprogesterone Screening Test for Congenital Adrenal Hyperplasia According to Birth Weight
- Establishment of the Separate Cutoff Values of 17-alpha-hydroxyprogesterone in Neonatal Screening Program for Congenital Adrenal Hyperplasia according to Birth Weight
- The clinical value of serum 17-hydroxyprogesterone levels for predicting LH surge in controlled ovarian hyperstimulation
- Analysis of Blood Spot 17-Hydroxyprogesterone Concentration According to Gestational Age and Birth Weight
- Serum 17-Hydroxyprogesterone Levels in Term and Preterm Infants