J Bone Metab.
2019 Feb;26(1):39-44. 10.11005/jbm.2019.26.1.39.
Impact on Bisphosphonate Persistence and Compliance: Daily Postprandial Administration
- Affiliations
-
- 1Department of Orthopaedic Surgery, Yeungnam University Medical Center, Daegu, Korea.
- 2Department of Orthopaedic Surgery, Soonchunhyang University Hospital Cheonan, Cheonan, Korea.
- 3Department of Orthopaedic Surgery, Soonchunhyang University Hospital Seoul, Seoul, Korea. huuy@schmc.ac.kr
- 4Division of Endocrinology and Metabolism, Department of Internal Medicine, Soonchunhyang University College of Medicine, Seoul, Korea.
- KMID: 2440644
- DOI: http://doi.org/10.11005/jbm.2019.26.1.39
Abstract
- BACKGROUND
Bisphosphonate (BP) is an effective drug for the prevention and treatment of osteoporosis. However, gastrointestinal distress caused by BP is a well-known side effect for low compliance. The aim of our study was to compare the 1-year persistence, compliance and T-scores between the aperitif medication group and the postprandial medication group.
METHODS
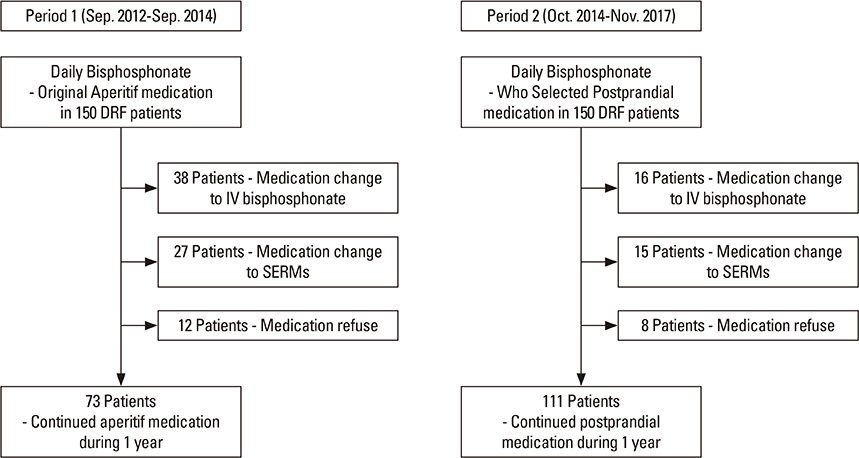
Three hundred patients were included in this study to determine their persistence and compliance with the prescribed daily BP (Maxmarvil®, alendronate 5 mg and calcitriol 0.5 µg; YuYu Pharm) following distal radius fractures. Patients in Group 1 (aperitif medication) were asked to adhere to the general guidelines for BPs before breakfast. Patients in Group 2 (postprandial medication) were recommended medication after breakfast. We compared the persistence and compliance of this daily BP therapy using the medication possession ratio (MPR) and T-scores between the 2 groups after 1 year.
RESULTS
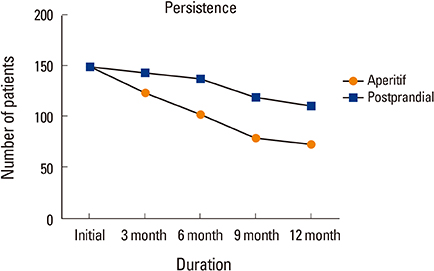
Bone mineral density in hip and lumbar spine was improved significantly in 2 groups (P < 0.001). Significant differences existed between 2 groups, including 73 of 150 patients (48.7%) in Group 1, and 111 of 150 patients (73.3%) in Group 2 for 1-year persistence (P=0.001). The mean MPR is 0.66 in Group 1 (range, 0.50-0.86) and 0.71 in Group 2 (range, 0.54-0.87). A significant difference was detected between the 2 groups (P=0.002).
CONCLUSIONS
Postprandial administration improved persistence and compliance with daily BP therapy, resulting in better clinical outcomes.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Comparison of Denosumab and Zoledronic Acid in Postmenopausal Women With Osteoporosis: Bone Mineral Density (BMD) and Trabecular Bone Score (TBS)
Taewook Kang, Si Young Park, Soon Hyuck Lee, Jong Hoon Park, Seung Woo Suh
J Korean Med Sci. 2022;37(13):e68. doi: 10.3346/jkms.2022.37.e68.
Reference
-
1. Curtis JR, Delzell E, Chen L, et al. The relationship between bisphosphonate adherence and fracture: is it the behavior or the medication? Results from the placebo arm of the fracture intervention trial. J Bone Miner Res. 2011; 26:683–688.
Article2. Ideguchi H, Ohno S, Hattori H, et al. Persistence with bisphosphonate therapy including treatment courses with multiple sequential bisphosphonates in the real world. Osteoporos Int. 2007; 18:1421–1427.
Article3. Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008; 11:44–47.
Article4. Briesacher BA, Andrade SE, Fouayzi H, et al. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008; 28:437–443.
Article5. Curtis JR, Westfall AO, Cheng H, et al. Benefit of adherence with bisphosphonates depends on age and fracture type: results from an analysis of 101,038 new bisphosphonate users. J Bone Miner Res. 2008; 23:1435–1441.
Article6. Nho JH, Lee YK, Ha YC, et al. Can alarming improve compliance with weekly bisphosphonate in patients with osteoporosis? J Bone Metab. 2016; 23:51–54.
Article7. van den Boogaard CH, Breekveldt-Postma NS, Borggreve SE, et al. Persistent bisphosphonate use and the risk of osteoporotic fractures in clinical practice: a database analysis study. Curr Med Res Opin. 2006; 22:1757–1764.
Article8. Gertz BJ, Holland SD, Kline WF, et al. Studies of the oral bioavailability of alendronate. Clin Pharmacol Ther. 1995; 58:288–298.
Article9. Hamilton B, McCoy K, Taggart H. Tolerability and compliance with risedronate in clinical practice. Osteoporos Int. 2003; 14:259–262.
Article10. Peng YL, Hu HY, Luo JC, et al. Alendronate, a bisphosphonate, increased upper and lower gastrointestinal bleeding: risk factor analysis from a nationwide population-based study. Osteoporos Int. 2014; 25:1617–1623.
Article11. Tosteson AN, Grove MR, Hammond CS, et al. Early discontinuation of treatment for osteoporosis. Am J Med. 2003; 115:209–216.
Article12. Mok JO, Jung CH, Kim CH, et al. Endoscopic comparison of alendronate alone and the enteric-coated alendronate with calcitriol combination in postmenopausal Korean females. Korean J Intern Med. 2013; 28:694–700.
Article13. Imaz I, Zegarra P, Gonzalez-Enriquez J, et al. Poor bisphosphonate adherence for treatment of osteoporosis increases fracture risk: systematic review and meta-analysis. Osteoporos Int. 2010; 21:1943–1951.
Article14. Weycker D, Macarios D, Edelsberg J, et al. Compliance with osteoporosis drug therapy and risk of fracture. Osteoporos Int. 2007; 18:271–277.
Article15. Siris ES, Harris ST, Rosen CJ, et al. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc. 2006; 81:1013–1022.
Article16. Cotte FE, Fardellone P, Mercier F, et al. Adherence to monthly and weekly oral bisphosphonates in women with osteoporosis. Osteoporos Int. 2010; 21:145–155.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Persistence and compliance with medication management in the treatment of overactive bladder
- Can Alarming Improve Compliance with Weekly Bisphosphonate in Patients with Osteoporosis?
- The Clinical Validity of Symptom-based Subgrouping of Irritable Bowel Syndrome According to the Rectal Sensory and Motor Function
- Which Bisphosphonate? It's the Compliance!: Decision Analysis
- Targeted Coaching to Improve Osteoporosis Therapy Adherence: A Single Arm Variation of the C-STOP Study