Korean J Radiol.
2019 Apr;20(4):580-588. 10.3348/kjr.2018.0564.
Impact of Energy and Access Methods on Extrahepatic Tumor Spreading and the Ablation Zone: An Ex vivo Experiment Using a Subcapsular Tumor Model
- Affiliations
-
- 1Department of Radiology, College of Medicine, Ewha Womans University, Seoul, Korea. kyongmd@ewha.ac.kr
- KMID: 2440481
- DOI: http://doi.org/10.3348/kjr.2018.0564
Abstract
OBJECTIVE
To evaluate the impact of energy and access methods on extrahepatic tumor spreading and the ablation zone in an ex vivo subcapsular tumor mimic model with a risk of extrahepatic tumor spreading.
MATERIALS AND METHODS
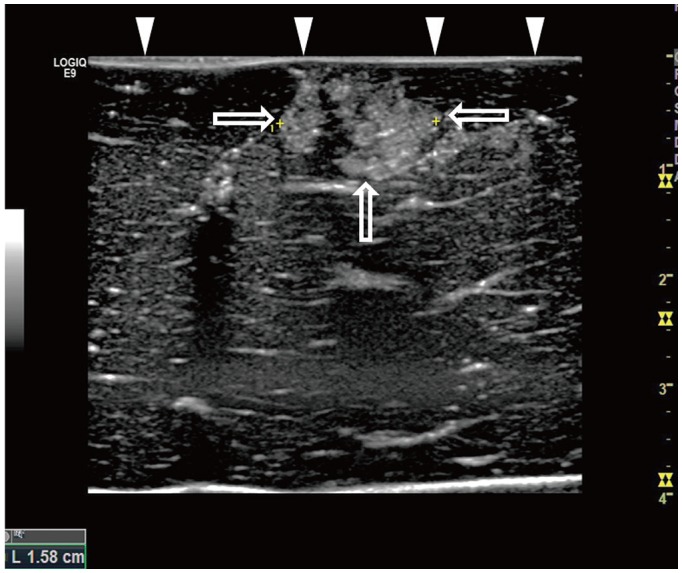
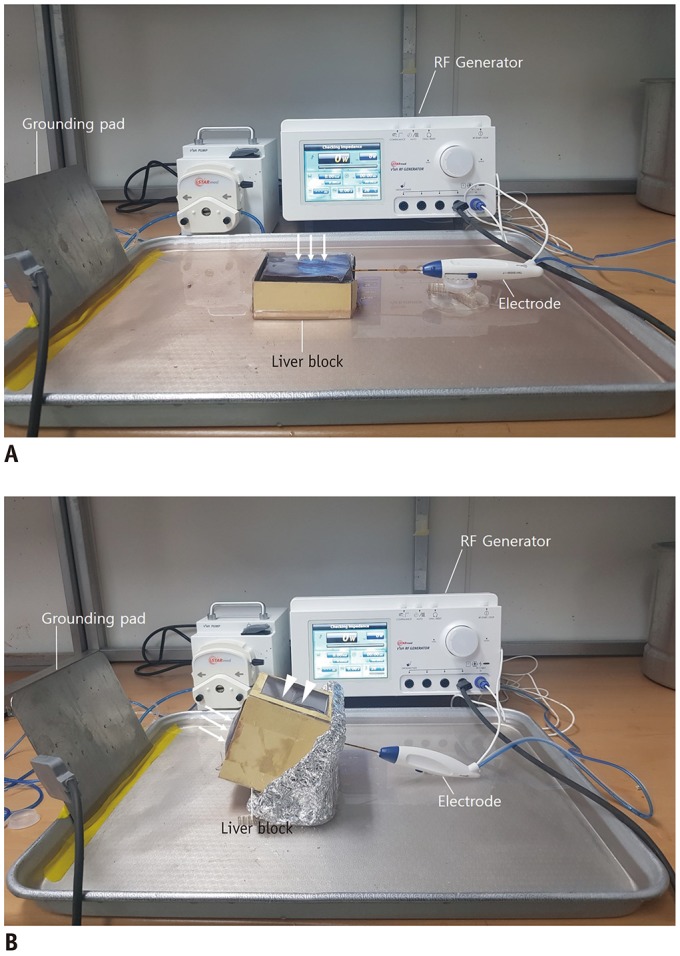
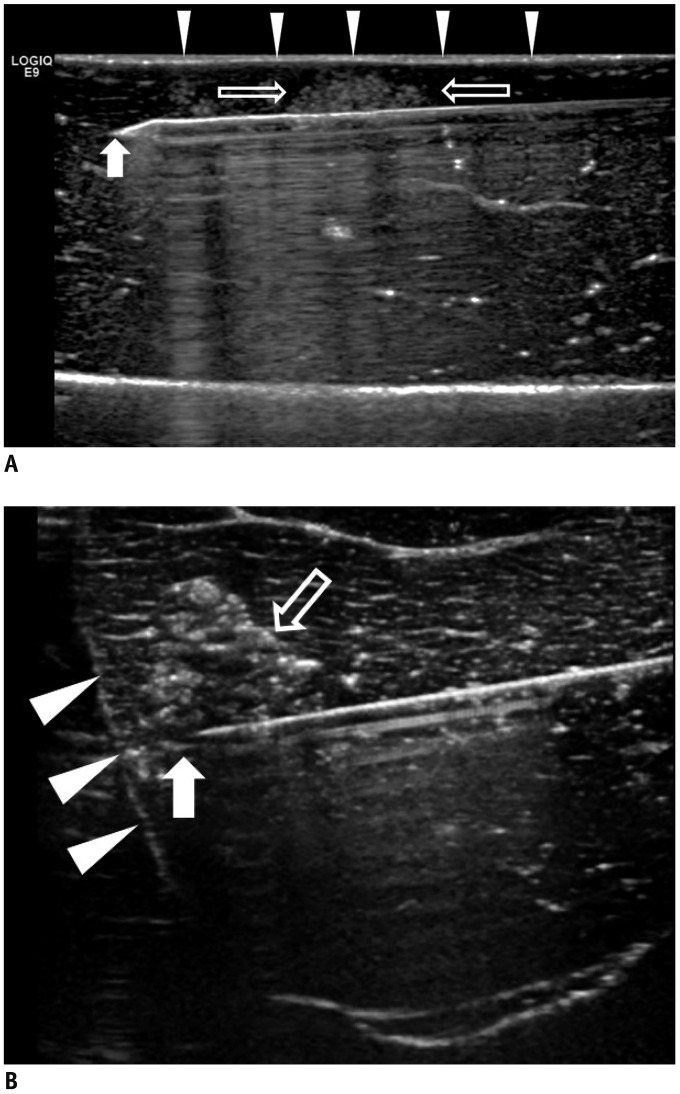
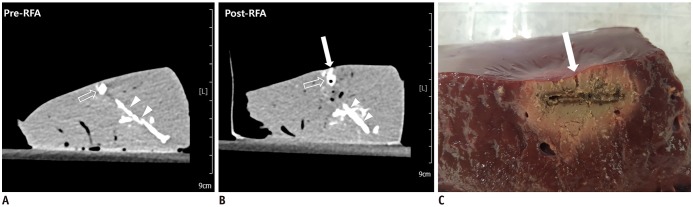
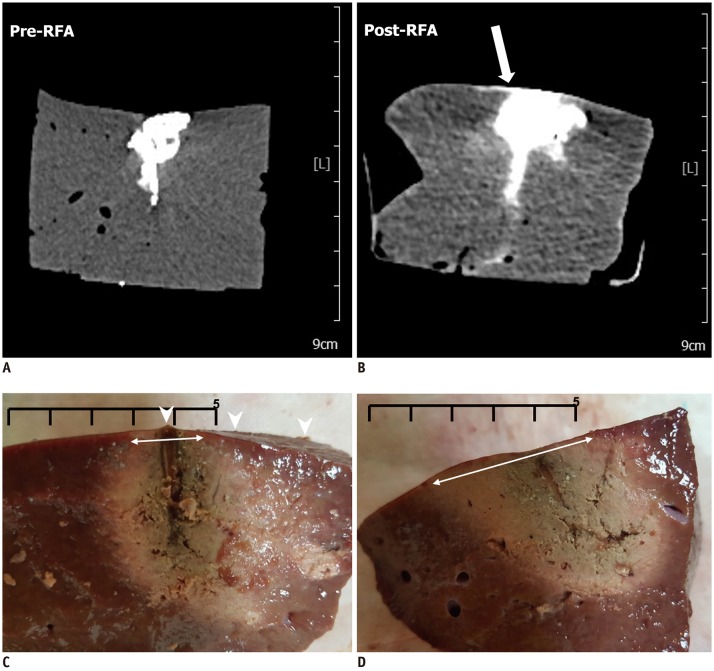
Forty-two tumor-mimics were created in bovine liver blocks by injecting a mixture of iodine contrast material just below the liver capsule. Radiofrequency (RF) ablations were performed using an electrode placed parallel or perpendicular to hepatic surface through the tumor mimic with low- and high-power protocols (groups 1 and 2, respectively). Computed tomography (CT) scans were performed before and after ablation. The presence of contrast leak on the hepatic surface on CT, size of ablation zone, and timing of the first roll-off and popping sound were compared between the groups.
RESULTS
With parallel access, one contrast leak in group 1 (1/10, 10%) and nine in group 2 (9/10, 90%) (p < 0.001) were identified on post-ablation CT. With perpendicular access, six contrast leaks were identified in each group (6/11, 54.5%). The first roll-off and popping sound were significantly delayed in group 1 irrespective of the access method (p = 0.002). No statistical difference in the size of the ablation zone of the liver specimen was observed between the two groups (p = 0.247).
CONCLUSION
Low-power RF ablation with parallel access is proposed to be effective and safe from extrahepatic tumor spreading in RF ablation of a solid hepatic tumor in the subcapsular location. Perpendicular placement of an electrode to the capsule is associated with a risk of extrahepatic tumor spreading regardless of the power applied.
MeSH Terms
Figure
Reference
-
1. Ahmed M, Brace CL, Lee FT Jr, Goldberg SN. Principles of and advances in percutaneous ablation. Radiology. 2011; 258:351–369. PMID: 21273519.
Article2. Llovet JM, Vilana R, Brú C, Bianchi L, Salmeron JM, Boix L, et al. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001; 33:1124–1129. PMID: 11343240.
Article3. Shirai K, Tamai H, Shingaki N, Mori Y, Moribata K, Enomoto S, et al. Clinical features and risk factors of extrahepatic seeding after percutaneous radiofrequency ablation for hepatocellular carcinoma. Hepatol Res. 2011; 41:738–745. PMID: 21699637.
Article4. Yu J, Liang P, Yu XL, Cheng ZG, Han ZY, Dong BW. Needle track seeding after percutaneous microwave ablation of malignant liver tumors under ultrasound guidance: analysis of 14-year experience with 1462 patients at a single center. Eur J Radiol. 2012; 81:2495–2499. PMID: 22137097.
Article5. Kim JW, Shin SS, Heo SH, Hong JH, Lim HS, Seon HJ, et al. Ultrasound-guided percutaneous radiofrequency ablation of liver tumors: how we do it safely and completely. Korean J Radiol. 2015; 16:1226–1239. PMID: 26576111.
Article6. Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003; 226:441–451. PMID: 12563138.
Article7. Teratani T, Yoshida H, Shiina S, Obi S, Sato S, Tateishi R, et al. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology. 2006; 43:1101–1108. PMID: 16628706.
Article8. Zhong-yi Z, Wei Y, Kun Y, Ying D, Wei W, Jung-Chieh L, et al. Needle track seeding after percutaneous radiofrequency ablation of hepatocellular carcinoma: 14-year experience at a single centre. Int J Hyperthermia. 2017; 33:454–458.
Article9. Baldan A, Marino D, DE Giorgio M, Angonese C, Cillo U, D'Alessandro A, et al. Percutaneous radiofrequency thermal ablation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2006; 24:1495–1501. PMID: 17081166.
Article10. Patel PA, Ingram L, Wilson ID, Breen DJ. No-touch wedge ablation technique of microwave ablation for the treatment of subcapsular tumors in the liver. J Vasc Interv Radiol. 2013; 24:1257–1262. PMID: 23885917.
Article11. Park SI, Kim IJ, Lee SJ, Shin MW, Shin WS, Chung YE, et al. Angled cool-tip electrode for radiofrequency ablation of small superficial subcapsular tumors in the liver: a feasibility study. Korean J Radiol. 2016; 17:742–749. PMID: 27587963.
Article12. Choe J, Kim KW, Kim YI, Chung JW, Huh J, Park J, et al. Feasibility of a low-power radiofrequency ablation protocol to delay steam popping. J Vasc Interv Radiol. 2016; 27:268–274. PMID: 26669701.
Article13. Kotoh K, Enjoji M, Arimura E, Morizono S, Kohjima M, Sakai H, et al. Scattered and rapid intrahepatic recurrences after radio frequency ablation for hepatocellular carcinoma. World J Gastroenterol. 2005; 11:6828. PMID: 16425391.
Article14. Lee DH, Lee JM. Recent advances in the image-Guided tumor ablation of liver malignancies: radiofrequency ablation with multiple electrodes, real-time multimodality fusion imaging, and new energy sources. Korean J Radiol. 2018; 19:545–559. PMID: 29962861.
Article15. Macatula TC, Lin CC, Lin CJ, Chen WT, Lin SM. Radiofrequency ablation for hepatocellular carcinoma: use of low vs maximal radiofrequency power. Br J Radiol. 2012; 85:e102–e109. PMID: 21427178.16. Scott DJ, Young WN, Watumull LM, Lindberg G, Fleming JB, Rege RV, et al. Development of an in vivo tumor-mimic model for learning radiofrequency ablation. J Gastrointest Surg. 2000; 4:620–625. PMID: 11307098.
Article17. Hildebrand P, Kleemann M, Roblick U, Mirow L, Bruch HP, Bürk C. Development of a perfused ex vivo tumor-mimic model for the training of laparoscopic radiofrequency ablation. Surg Endosc. 2007; 21:1745–1749. PMID: 17332954.
Article18. Kim JH, Kim PN, Won HJ, Shin YM. Percutaneous radiofrequency ablation using internally cooled wet electrodes for the treatment of hepatocellular carcinoma. AJR Am J Roentgenol. 2012; 198:471–476. PMID: 22268196.
Article19. Kotoh K, Nakamuta M, Morizono S, Kohjima M, Arimura E, Fukushima M, et al. A multi-step, incremental expansion method for radio frequency ablation: optimization of the procedure to prevent increases in intra-tumor pressure and to reduce the ablation time. Liver Int. 2005; 25:542–547. PMID: 15910491.
Article20. Kim YJ, Raman SS, Yu NC, Busuttil RW, Tong M, Lu DS. Radiofrequency ablation of hepatocellular carcinoma: can subcapsular tumors be safely ablated? AJR Am J Roentgenol. 2008; 190:1029–1034. PMID: 18356451.
Article21. Pereira PL, Trübenbach J, Schenk M, Subke Jr, Kroeber S, Schaefer I, et al. Radiofrequency ablation: in vivo comparison of four commercially available devices in pig livers. Radiology. 2004; 232:482–490. PMID: 15286318.
Article22. Cua IHY, Lin CC, Lin CJ, Chen WT, Hsu CW, Chen YC, et al. Treatment of hepatocellular carcinoma using internally cooled electrodes. A prospective comparison of modified automated vs. manual pulsed radiofrequency algorithms. Oncology. 2007; 72:76–82. PMID: 18087186.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Microwave Ablation Power and Antenna Approach on Tumor Seeding: An Ex Vivo Subcapsular Tumor Model Study
- Ablative Outcomes of Various Energy Modes for No-Touch and Peripheral Tumor-Puncturing Radiofrequency Ablation: An Ex Vivo Simulation Study
- Bipolar Radiofrequency Ablation Using Dual Internally Cooled Wet Electrodes: Experimental Study in Ex Vivo Bovine Liver
- Feasibility of Saline Infusion on the Liver Surface during Radiofrequency Ablation of Subcapsular Hepatic Tumor: An Experimental Study
- Does Artificial Ascites Induce the Heat-Sink Phenomenon during Percutaneous Radiofrequency Ablation of the Hepatic Subcapsular Area?: an in vivo Experimental Study Using a Rabbit Model