Ann Dermatol.
2019 Apr;31(2):217-220. 10.5021/ad.2019.31.2.217.
A Permanent Hair Loss in a Patient with Hypersensitivity to Intralesional Triamcinolone Injection
- Affiliations
-
- 1Department of Dermatology and Cutaneous Biology Research Institute, Yonsei University College of Medicine, Seoul, Korea. dykim@yuhs.ac
- 2Yonsei Leeand Skin Clinic, Seoul, Korea.
- KMID: 2439070
- DOI: http://doi.org/10.5021/ad.2019.31.2.217
Abstract
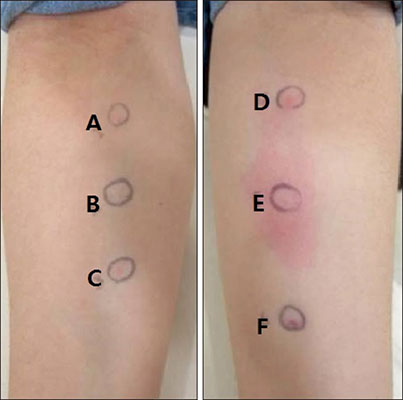
- Despite multiple possible side effects, mesotherapy with a diverse mixture of unapproved products has been performed for the treatment of hair loss. In this case report, we present a rare case of permanent hair loss due to an allergic reaction from a mesotherapy mixture including triamcinolone acetonide. The patient showed a positive intradermal allergic skin test result for triamcinolone and therefore was diagnosed with scarring alopecia due to delayed type IV hypersensitivity to corticosteroid. This case study suggests that dermatologists should always be fully aware that both IgE-mediated and non-IgE-mediated hypersensitivity reactions from the corticosteroids as well as their mesotherapy excipients are possible in an effort to prevent irreversible adverse event from the mesotherapy.
Keyword
MeSH Terms
Figure
Reference
-
1. Laisuan W, Wongsa C, Dchapaphapeaktak N, Tongdee M, Chatmapanrangsee J, Rerkpattanapipat T. Anaphylaxis following intralesional triamcinolone acetonide (Kenacort) injection. Asia Pac Allergy. 2017; 7:115–118.
Article2. Laberge L, Pratt M. Immediate and delayed hypersensitivity to systemic corticosteroids: 2 case reports. Dermatitis. 2012; 23:288–290.3. Räsänen L, Hasan T. Allergy to systemic and intralesional corticosteroids. Br J Dermatol. 1993; 128:407–411.
Article4. Saff DM, Taylor JS, Vidimos AT. Allergic reaction to intralesional triamcinolone acetonide: a case report. Arch Dermatol. 1995; 131:742–743.
Article5. Comaish S. A case of hypersensitivity to corticosteroids. Br J Dermatol. 1969; 81:919–925.
Article6. Kilpiö K, Hannuksela M. Corticosteroid allergy in asthma. Allergy. 2003; 58:1131–1135.
Article7. Browne F, Wilkinson SM. Effective prescribing in steroid allergy: controversies and cross-reactions. Clin Dermatol. 2011; 29:287–294.
Article8. Bircher AJ, Bigliardi P, Zaugg T, Mäkinen-Kiljunen S. Delayed generalized allergic reactions to corticosteroids. Dermatology. 2000; 200:349–351.
Article9. Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989; 121:27–34.
Article10. Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000; 55:698–704.
Article11. Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermatitis. 2012; 66:38–45.
Article12. Pratt MD, Mufti A, Lipson J, Warshaw EM, Maibach HI, Taylor JS, et al. Patch test reactions to corticosteroids: retrospective analysis from the North American Contact Dermatitis Group 2007-2014. Dermatitis. 2017; 28:58–63.
Article13. Montoro J, Valero A, Elices A, Rubira N, Serra-Baldrich E, Amat P, et al. Anaphylactic shock after intra-articular injection of carboxymethylcellulose. Allergol Immunopathol (Madr). 2000; 28:332–333.14. Al Hadithy A, van Maaren M, Vermes A. Anaphylactic reactions following Kenacort-A® injection: carboxymethylcellulose is involved once again. Contact Dermatitis. 2011; 64:179–180.
Article15. Bigliardi PL, Izakovic J, Weber JM, Bircher AJ. Anaphylaxis to the carbohydrate carboxymethylcellulose in parenteral corticosteroid preparations. Dermatology. 2003; 207:100–103.
Article16. Duque-Estrada B, Vincenzi C, Misciali C, Tosti A. Alopecia secondary to mesotherapy. J Am Acad Dermatol. 2009; 61:707–709.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Optimal method of intralesional corticosteroid injection in the treatment of alopecia areata
- Intralesional Injection of Verapamil Only and Verapamil and Serial Triamcinolone Acetonide in Peyronie's Disease
- Giant Infantile Hemangioma Treated with Beta-blocker with Intermittent Triamcinolone Intralesional Injection
- Intralesional Injection of Triamcinolone for Urethral Stricture after Visual Urethrotomy
- Branch-shaped Cutaneous Hypopigmentation and Atrophy after Intralesional Triamcinolone Injection