J Breast Cancer.
2017 Sep;20(3):304-309. 10.4048/jbc.2017.20.3.304.
Follow-up Outcomes of Benign Pathology Initially Assigned as Breast Imaging Reporting and Data System Category 4A and 3
- Affiliations
-
- 1Center for Breast Cancer, Research Institute and Hospital, National Cancer Center, Goyang, Korea. kokr@ncc.re.kr
- 2Cancer Prevention and Early Detection Center, National Cancer Center, Goyang, Korea.
- 3National Cancer Control Institute, National Cancer Center, Goyang, Korea.
- 4Department of Radiology, Moores Cancer Center, UC San Diego Health System, La Jolla, USA.
- KMID: 2438998
- DOI: http://doi.org/10.4048/jbc.2017.20.3.304
Abstract
- PURPOSE
This retrospective study investigated if the initially assigned category 4A or 3 in concordant benign lesions, after ultrasound (US)-guided core needle biopsy, could affect follow-up compliance.
METHODS
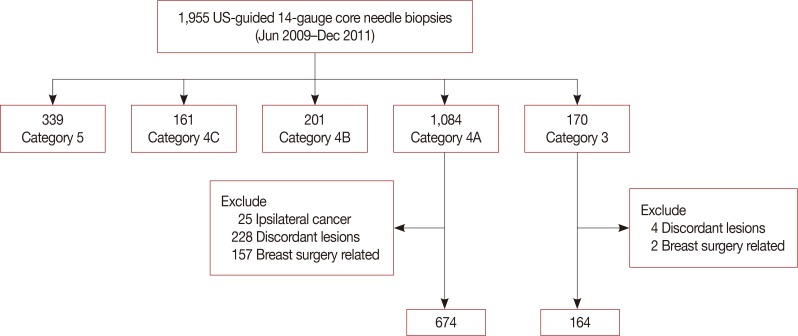
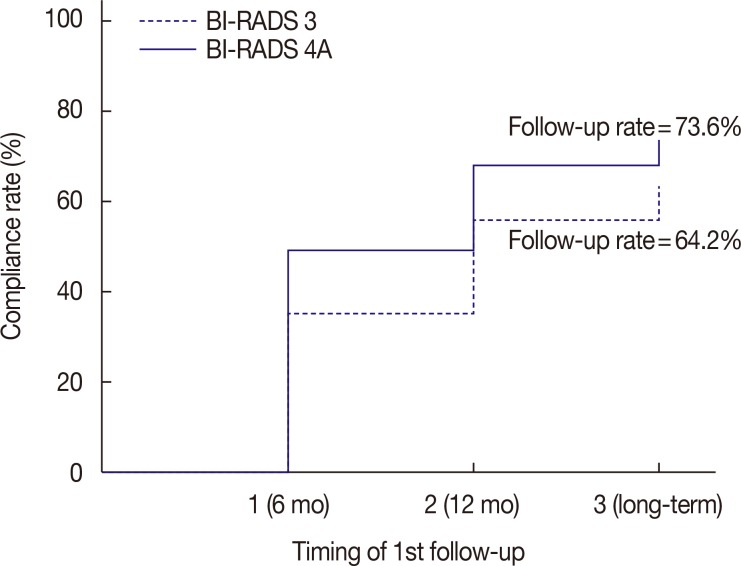
Eight hundred thirty-eight concordant benign lesions, after core needle biopsy (674, initial category 4A group and 164, category 3 group) and follow-up US, were included in our study. If an immediate surgical excision"”a surgical excision before the next follow-up"”exists, those cases with pathologic reports were collected. Statistical comparisons for the result of follow-up US compliance, additional biopsy, and malignant rates among 6-month, 12-month, and long-term intervals were performed by using the chi-square test. The log-rank test was used to compare compliance rates in the timing of first follow-up between the two groups, with a significance level of 0.05.
RESULTS
The number of immediate surgical excision was higher in the category 4A group (11.1%) than in the category 3 group (6.1%); only one cancer was found in the category 4A group. The patients' compliance rate in a 6-month follow-up showed an increase (p=0.003) in the category 4A group. The additional biopsy rate was higher in the initial category 4A group (10.9%) than in the category 3 group (1.9%) with statistical significance (p=0.001); four cancers were found on additional biopsy in the category 4 group. No cancer was detected in the initial category 3 group, both on immediate surgical excision and follow-up.
CONCLUSION
The initial category 4A or 3 of the Breast Imaging Reporting and Data System could be a significant factor that affects immediate surgical excision and follow-up compliance. Cancers were detected only in the initial category 4A group of concordant benign lesions. More attention should be paid to the concordant benign lesions from the initial category 4A group than from the category 3 group.
MeSH Terms
Figure
Reference
-
1. American College of Radiology. Breast Imaging Reporting And Data System (BI-RADS®). 4th ed. Reston: American College of Radiology;2003.2. Parikh J, Tickman R. Image-guided tissue sampling: where radiology meets pathology. Breast J. 2005; 11:403–409. PMID: 16297084.
Article3. Youk JH, Jung I, Kim EK, Kim MJ, Son EJ, Moon HJ, et al. US follow-up protocol in concordant benign result after US-guided 14-gauge core needle breast biopsy. Breast Cancer Res Treat. 2012; 132:1089–1097. PMID: 22218886.
Article4. Lee CH, Philpotts LE, Horvath LJ, Tocino I. Follow-up of breast lesions diagnosed as benign with stereotactic core-needle biopsy: frequency of mammographic change and false-negative rate. Radiology. 1999; 212:189–194. PMID: 10405741.
Article5. Marcon M, Frauenfelder T, Becker AS, Dedes KJ, Boss A. First ultrasound diagnosis of BI-RADS 3 lesions in young patients: can 6-months follow-up be sufficient to assess stability. Eur J Radiol. 2017; 89:226–233. PMID: 28267544.
Article6. Sickles EA. Probably benign breast lesions: when should follow-up be recommended and what is the optimal follow-up protocol? Radiology. 1999; 213:11–14. PMID: 10540635.
Article7. Salkowski LR, Fowler AM, Burnside ES, Sisney GA. Utility of 6-month follow-up imaging after a concordant benign breast biopsy result. Radiology. 2011; 258:380–387. PMID: 21079199.
Article8. Liberman L, Drotman M, Morris EA, LaTrenta LR, Abramson AF, Zakowski MF, et al. Imaging-histologic discordance at percutaneous breast biopsy. Cancer. 2000; 89:2538–2546. PMID: 11135213.
Article9. Chae EY, Cha JH, Shin HJ, Choi WJ, Kim HH. Reassessment and follow-up results of BI-RADS category 3 lesions detected on screening breast ultrasound. AJR Am J Roentgenol. 2016; 206:666–672. PMID: 26901026.
Article10. Graf O, Helbich TH, Hopf G, Graf C, Sickles EA. Probably benign breast masses at US: is follow-up an acceptable alternative to biopsy? Radiology. 2007; 244:87–93. PMID: 17581897.
Article11. Raza S, Chikarmane SA, Neilsen SS, Zorn LM, Birdwell RL. BI-RADS 3, 4, and 5 lesions: value of US in management--follow-up and outcome. Radiology. 2008; 248:773–781. PMID: 18647850.
Article12. Borders MH, Cheng L, Fitzpatrick KA, Krupinski EA. Patient compliance in the setting of BI-RADS category 3: what factors impact compliance with short-term follow-up recommendations? Breast J. 2017; 23:77–82. PMID: 27859923.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Follow-up Outcomes of Benign Pathology Initially Assigned as Breast Imaging Reporting and Data System Category 4A and 3
- Usefulness of ultrasound elastography in reducing the number of Breast Imaging Reporting and Data System category 3 lesions on ultrasonography
- Predictive Value of BI-RADS Category 4A and 4B Lesions Detected on Breast Ultrasonography: Single Center Experience
- Outcomes of US BI-RADS 4A, 4B, and 4C Lesions
- Categorization and Evaluation of Usefulness of Breast Lesions with using Ultrasound BI-RADS (Breast Imaging Reporting and Data system)