Nat Prod Sci.
2018 Dec;24(4):266-271. 10.20307/nps.2018.24.4.266.
Five New Stilbenes from the Stem Bark of Artocarpus communis
- Affiliations
-
- 1Molecular Targets Program, Center for Cancer Research, National Cancer Institute, Frederick, Maryland, USA, 21702. gustafki@mail.nih.gov
- 2Current Address: The Ferrier Research Institute of Victoria, University of Wellington, Lower Hut, New Zealand.
- 3Current Address: Institute of Environmental Science and Research Ltd., Kenepuru Science Centre, Porirua, New Zealand.
- 4Basic Science Program, Leidos Biomedical Research, Inc, Frederick National Laboratory for Cancer Research sponsored by the National Cancer Institute, Frederick, Maryland, USA 21702.
- 5Current Address: Diagnostic Biomarkers and Technology Branch, Cancer Diagnosis Program, National Cancer Institute, Rockville, Maryland, USA 20850-9728.
- KMID: 2432422
- DOI: http://doi.org/10.20307/nps.2018.24.4.266
Abstract
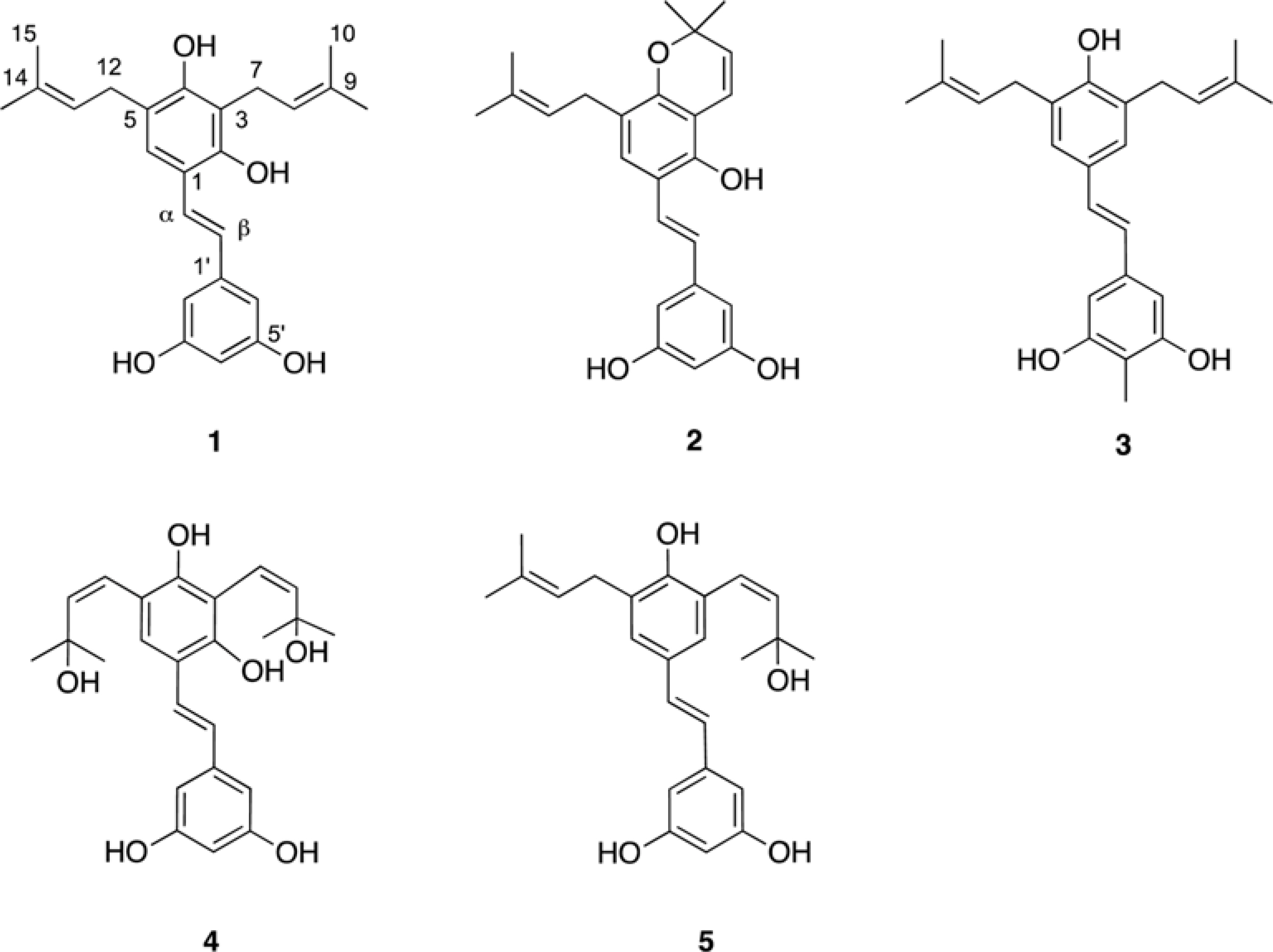
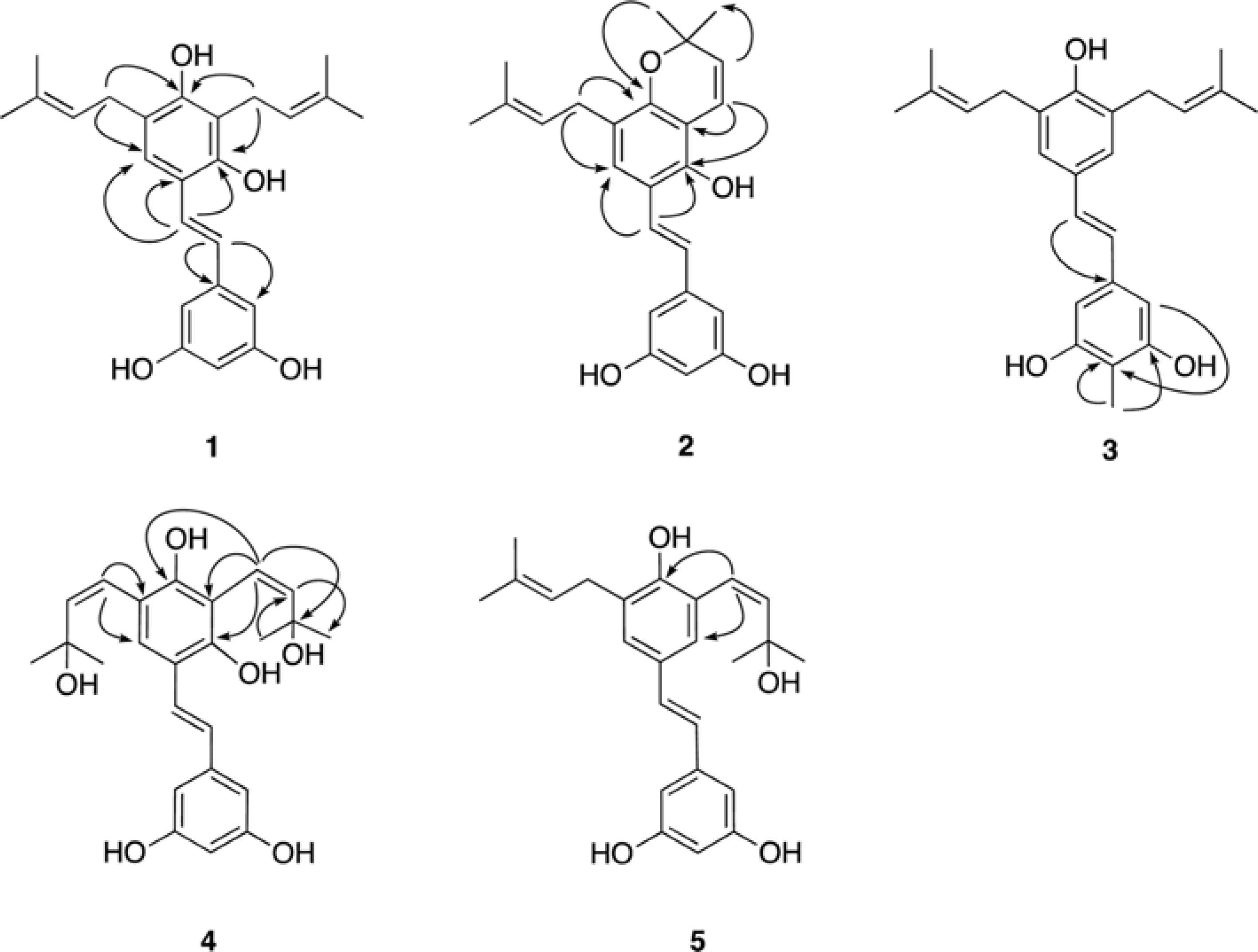
- Five new prenylated stilbenes (1 - 5), along with the known compounds cudraflavone C, trans-4-isopentenyl-3,5,2"²,4"²-terahydroxystilbene, trans-4-(3-methyl-E-but-1-enyl)-3,5,2"²,4"²-tetrahydroxystilbene, pannokin G, cycloartobiloxanthone, artonin P, morusin, artocarpin, artonin E, kuwanon C, artobiloxanthone, and artoindonesianin C (6 - 17) were isolated from the stem bark of the tropical tree Artocarpus communis. The structures were established by NMR spectroscopic analysis, MS studies, and comparison with spectral data reported in the literature.
Keyword
MeSH Terms
Figure
Reference
-
(1). Jagtap U. B., Bapat V. A. J.Ethnopharmacol. 2010; 129:142–166.(2). Sikarwar M. S., Hui B. J., Subramaniam K., Valeisamy B. D., Yean L. K., Balaji K. J.Appl. Pharm. Sci. 2014; 4:91–97.(3). McKee T. C., Rabe D., Bokesch H. R., Grkovic T., Whitson E. L., Diyabalanage T., Van Wyk A. W. W., Marcum S. R., Gardella R. S., Gustafson K. R., Linehan W. M., McMahon J. B., Bottaro D. P. J.Nat. Prod. 2012; 75:1632–1636.(4). Löfstedt T., Fredlund E., Holmquist-Mengelbier L., Pietras A., Ovenberger M., Poelinger L., Pahlman S.Cell Cycle. 2007; 8:919–926.(5). Bokesch H. R., Gardella R. S., Rabe D. C., Bottaro D. P., Linehan W. M., McMahon J. B., McKee T. C.Chem. Pharm. Bull. 2011; 59:1178–1179.(6). McCloud T. G.Molecules. 2010; 15:4526–4563.(7). Toume K., Habu T., Arai M. A., Koyano T., Kowithayakorn T., Ishibashi M. J.Nat. Prod. 2015; 78:103–110.(8). Pacher T., Seger C., Engelmeier D., Vajrodaya S., Hofer O., Greger H. J.Nat. Prod. 2002; 65:820–827.(9). Kostecki K., Engelmeier D., Pacher T., Hofer O., Vajrodaya S., Greger H.Phytochemistry. 2004; 65:99–106.(10). Hano Y.Heterocycles. 1990; 31:1339–1344.(11). Takasugi M, Muñoz L., Masamune T., Shirata A., Takahashi K.Chem. Lett. 1978; 7:1241–1242.(12). Boonlaksiri C., Oonanant W., Kongsaeree P., Kittakoop P., Tanticharoen M., Thebtaranonth Y.Phytochemistry. 2000; 54:415–417.(13). Sultanbawa M. U. S., Surendrakumar S.Phytochemistry. 1989; 28:599–605.(14). Hano Y., Inami R., Nomura T.Heterocycles. 1993; 35:1341–1350.(15). Nomura T., Fukai T., Yamada S., Katayanagi M.Chem. Pharm. Bull. 1976; 24:2898–2900.(16). Nomura T., Fukai T.Heterocycles. 1979; 12:1289–1295.(17). Lin C. N., Lu C. M., Huang P. L.Phytochemistry. 1995; 39:1447–1451.1451.(18) Sato M.., Fujiwara S.., Tsuchiya H.., Fujii T.., Iinuma M.., Tosa H.., Ohkawa Y. J.Ethnopharmacol. 1996. 54:171–176.(19). Hano Y., Yamagami Y., Kobayashi M., Isohata R., Nomura T.Heterocycles. 1990; 31:877–882.(20). Nomura T., Fukai T., Katayanagi M.Chem. Pharm. Bull. 1977; 25:529–532.(21). Makmur L., Syamsurizal S., Tukiran T., Achmad S. A., Aimi N., Hakim E. H., Kitajima M., Takayama H. J.Nat. Prod. 2000; 63:243–244.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- First Report of Stem Rot on Asiatic Dayflower (Commelina communis L.) Caused by Sclerotium rolfsii in Korea
- Sesbagrandiflorain F, a New 2-Arylbenzofuran from the Stem Bark of Sesbania grandiflora L.
- First Report of Stemonitis splendens Rostaf Causing Bark Decay of Oak Logs Used for Shiitake Cultivation in Korea
- Biocontrol Effect of Gliocladium virens G1 and Soil Amendment on Astragal Stem Rot Caused by Rhizoctonia solani
- Simultaneous HPLC Analysis of Three Flavonoids in the Extracts of Artocarpus heterophyllus Heartwoods