Nat Prod Sci.
2018 Dec;24(4):253-258. 10.20307/nps.2018.24.4.253.
New Meroterpenoids from a Penicillium sp. Fungus
- Affiliations
-
- 1Natural Products Research Institute, College of Pharmacy, Seoul National University, San 56-1, Sillim, Gwanak, Seoul 151-742, Korea. shinj@snu.ac.kr
- 2Department of Agricultural Biotechnology, College of Agricultural and Life Science, Seoul National University, San 56-1, Sillim, Gwanak, Seoul 151-921, Korea. ohkibong@snu.ac.kr
- KMID: 2432420
- DOI: http://doi.org/10.20307/nps.2018.24.4.253
Abstract
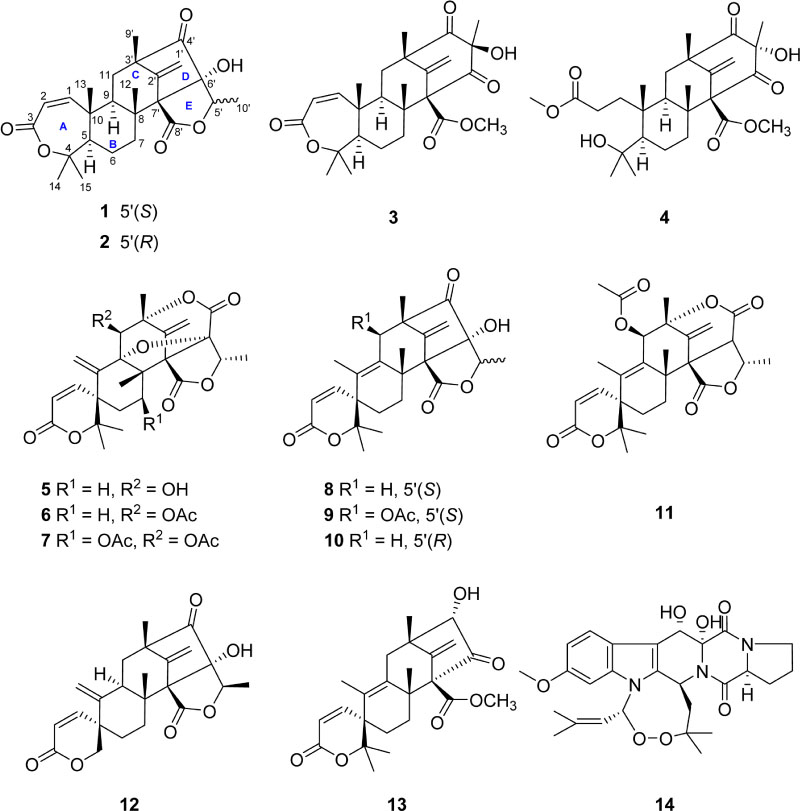
- Two meroterpenoids (1 and 2) along with twelve known compounds (3 - 14) were isolated from the culture broth of a Penicillium sp. fungus collected from Chuja-do, Korea. Based on the results of a combination of spectroscopic analyses, the new compounds, preaustinoids E (1) and F (2), were determined to be epimeric austin-type penta-cyclic lactones.
Keyword
MeSH Terms
Figure
Reference
-
1. Aly AH, Debbab A, Proksch P. Fungal Divers. 2011; 50:3–19.2. Wiemann P, Keller NP. J Ind Microbiol Biotechnol. 2014; 41:301–313.3. Matsuda Y, Abe I. Nat Prod Rep. 2016; 33:26–53.4. Schueffler A, Anke T. Nat Prod Rep. 2014; 31:1425–1448.5. Chexal KK, Springer JP, Clardy J, Cole RJ, Kirksey JW, Dorner JW, Cutler HG, Strawter BJ. J Am Chem Soc. 1976; 98:6748–6750.6. Geris R, Simpson T. Nat Prod Rep. 2009; 26:1063–1094.7. Matsuda Y, Awakawa T, Wakimoto T, Abe I. J Am Chem Soc. 2013; 135:10962–10965.8. Matsuda Y, Iwabuchi T, Fujimoto T, Awakawa T, Nakashima Y, Mori T, Zhang H, Hayashi F, Abe I. J Am Chem Soc. 2016; 138:12671–12677.9. Matsuda Y, Awakawa T, Mori T, Abe I. Curr Opin Chem Biol. 2016; 31:1–7.10. Mosmann T. J Immunol Methods. 1983; 65:55–63.11. Ulukaya E, Ozdikicioglu F, Oral AY, Demirci M. Toxicol In Vitro. 2008; 22:232–239.12. Johansson M, Karlsson L, Wennergren M, Jansson T, Powell TL. J Clin Endocrinol Metab. 2003; 88:2831–2837.13. Oh KB, Kim SH, Lee J, Cho WJ, Lee T, Kim S. J Med Chem. 2004; 47:2418–2421.14. Lee HS, Lee TH, Yang SH, Shin HJ, Shin J, Oh KB. Bioorg Med Chem Lett. 2007; 17:2483–2486.15. Oh KB, Lee JH, Chung SC, Shin J, Shin HJ, Kim HK, Lee HS. Bioorg Med Chem Lett. 2008; 18:104–108.16. dos Santos RM, Rodrigues-Fo E. Z Naturforsch C. 2003; 58:663–669.17. Fill TP, Pereira GK, Geris dos Santos RM, Rodrigues-Fo E. Z Naturforsch. 2007; 62b:1035–1044.18. Lo HC, Entwistle R, Guo CJ, Ahuja M, Szewczyk E, Hung JH, Chiang YM, Oakley BR, Wang CC. J Am Chem Soc. 2012; 134:4709–4720.19. Arunpanichlert J, Rukachaisirikul V, Phongpaichit S, Supaphon O, Sakayaroj J. Tetrahedron. 2015; 71:882–888.20. Hayashi H, Mukaihara M, Murao S, Arai M, Lee AY, Clardy J. Biosci Biotech Biochem. 1994; 58:334–338.21. Fayos J, Lokensgard D, Clardy J, Cole RJ, Kirksey JW. J Am Chem Soc. 1974; 96:6785–6787.22. Simpson TJ, Stenzel DJ, Bartlett AJ, O'Brien E, Holker JSE. J Chem Soc Perkin Trans 1. 1982; 2687–2692.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Disseminated Penicillium marneffei Infection in a Liver Transplant Recipient
- Three New Records of Penicillium Species Isolated from Insect Specimens in Korea
- Penidioxolanes A and B, 1,3-Dioxolane Containing Azaphilone Derivatives from Marine-derived Penicillium sp. KCB12C078
- Penicillium ulleungdoense sp. nov. from Ulleung Island in Korea
- Penicilliosis in AIDS