Nat Prod Sci.
2015 Dec;21(4):231-236. 10.20307/nps.2015.21.4.231.
Penidioxolanes A and B, 1,3-Dioxolane Containing Azaphilone Derivatives from Marine-derived Penicillium sp. KCB12C078
- Affiliations
-
- 1Chemical Biology Research Center, Korea Research Institute of Bioscience and Biotechnology, Chungbuk 363-883, Korea. jsahn@kribb.re.kr, jangjh@kribb.re.kr
- 2Department of Biomolecular Science, University of Science and Technology, Daejeon 305-333, Korea.
- 3RIKEN-KRIBB Joint Research Unit, Global Research Cluster, RIKEN, Saitama 351-0198, Japan.
- 4Chemical Biology Research Group, RIKEN CSRS, Saitama 351-0198, Japan.
- 5Microbial Resources Center, KRIBB, Daejeon 306-809, Korea.
- KMID: 2312901
- DOI: http://doi.org/10.20307/nps.2015.21.4.231
Abstract
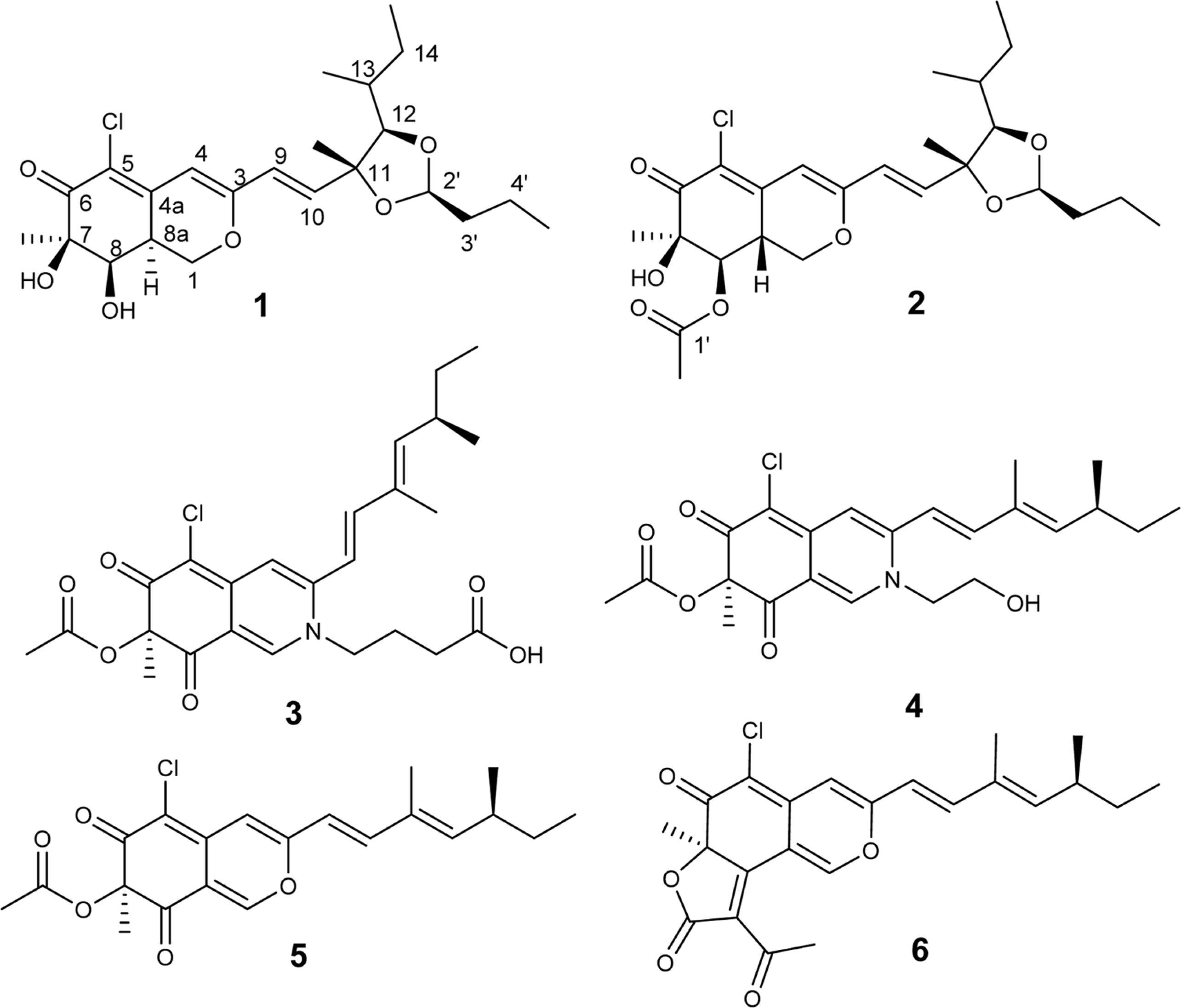
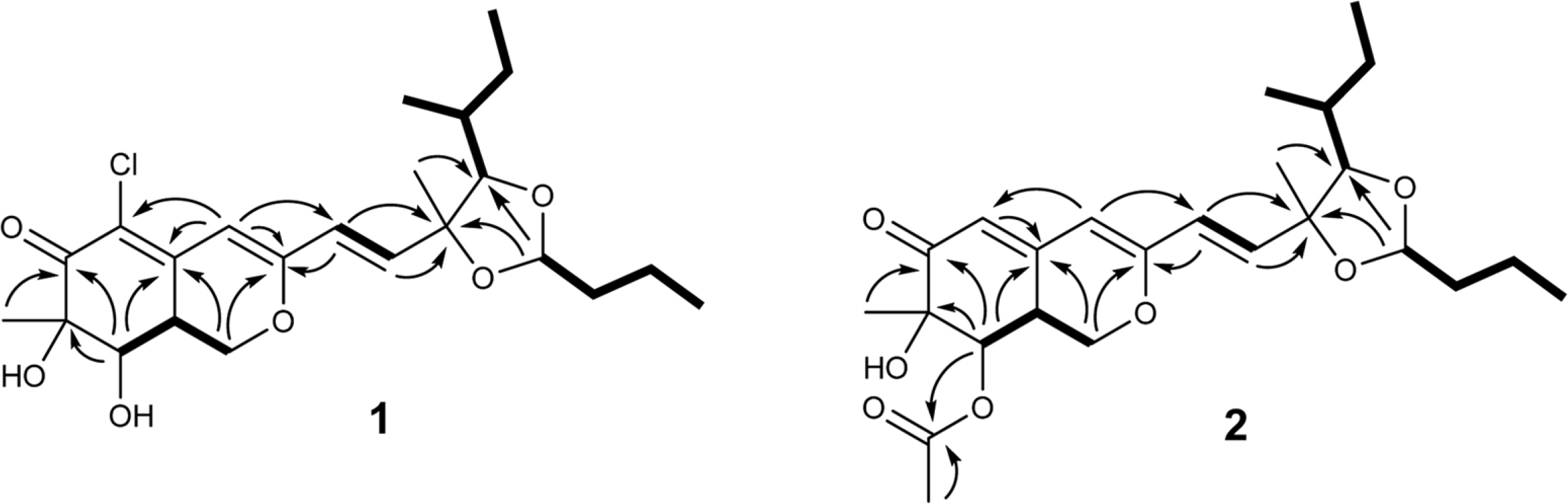
- Two new azaphilone derivatives containing 1,3-dioxolane moiety, penidioxolanes A (1) and B (2), were isolated from marine-derived fungus Penicillium sp. KCB12C078, together with four known compounds (3-6) by chemical investigation. Compounds 1 - 6 were isolated by combination of silica gel, ODS column chromatography and preparative HPLC. Their structures were determined by analysis of spectroscopic data including 1D-, 2D-NMR, and MS techniques. The isolates were evaluated against cancer cell growth inhibition effects and antimicrobial activity.
Figure
Reference
-
(1). Bérdy J. J.Antibiot. 2005; 58:1–26.(2). Fenical W., Jensen P. R.Nat. Chem. Biol. 2006; 2:666–673.(3). Faulkner D. J.Nat. Prod. Rep. 1998; 15:113–158.(4). Satpute S. K., Banat I. M., Dhakephalkar P. K., Banpurkar A. G., Chopade B. A.Biotechnol. Adv. 2010; 28:436–450.(5). Osmanova N., Schultze W., Ayoub N.Phytochem. Rev. 2010; 9:315–342.(6). Gao J. M., Yang S. X., Qin J. C.Chem. Rev. 2013; 113:4755–4811.(7). Quang D. N., Hashimoto T., Fournier J., Stadler M., Radulovi cí N., Asakawa Y.Tetrahedron. 2005; 61:1743–1748.(8). Kanokmedhakul S., Kanokmedhakul K., Nasomjai P., Louangsy-souphanh S., Soytong K., Isobe M., Kongsaeree P., Prabpai S., Suksamrarn A. J.Nat. Prod. 2006; 69:891–895.(9). Yu B. Z., Zhang G. H., Du Z. Z., Zheng Y. T., Xu J. C., Luo X. D.Phytochemistry. 2008; 69:2523–2526.(10). Quang D. N., Hashimoto T., Tanaka M., Stadler M., Asakawa Y.Phytochemistry. 2004; 65:469–473.(11). Li J. J., Shang X. Y., Li L. L., Liu M. T., Zheng J. Q., Jin Z. L.Molecules. 2010; 15:1958–1966.(12). Dong J., Zhou Y., Li R., Zhou W., Li L., Zhu Y., Huang R., Zhang K.FEMS. Microbiol. Lett. 2006; 264:65–69.(13). Yasukawa K., Takahashi M., Natori S., Kawai K., Yamazaki M., Takeuchi M., Takido M.Oncology. 1994; 51:108–112.(14). Michael A. P., Grace E. J., Kotiw M., Barrow R. A.Aust. J. Chem. 2003; 56:13–15.(15). Arai N., Shiomi K., Tomoda H., Tabata N., Yang D. J., Masuma R., Kawakubo T., Omura S. J.Antibiot. 1995; 48:696–702.(16). Curtin T. P., Reilly J.Biochem. J. 1940; 34:1419–1421.(17). Holker J. S. E., Ross W. J., Staunton J., Whalley W. B. J.Chem. Soc. 1962; 4150–4154.(18). Gray R. W., Whalley W. B.Chem. Commun. 1970; 12:762.(19). Yang D. J., Tomoda H., Tabata N., Masuma R., Omura S. J.Antibiot. 1996; 49:223–229.(20). Matsuzaki K., Tanaka H., Omura S. J.Antibiot. 1995; 48:708–713.(21). Arunpanichlert J., Rukachaisirikul V., Sukpondma Y., Phongpaichit S., Tewtrakul S., Rungjindamai N., Sakayaroj J.Chem. Pharm. Bull. 2010; 58:1033–1036.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Penicillium vietnamense sp. nov., the First Novel Marine Fungi Species Described from Vietnam with a Unique Conidiophore Structure and Molecular Phylogeny of Penicillium Section Charlesia
- Three Unrecorded Species Belonging to Penicillium Section Sclerotiora from Marine Environments in Korea
- Viridicatol from Marine-derived Fungal Strain Penicillium sp. SF-5295 Exerts Anti-inflammatory Effects through Inhibiting NF-kappaB Signaling Pathway on Lipopolysaccharide-induced RAW264.7 and BV2 Cells
- A New Record of Penicillium antarcticum from Marine Environments in Korea
- Calyxaprenols A-D, New Merohexaprenoid Metabolites from the Marine Sponge Calyx sp.