Ann Lab Med.
2019 May;39(3):252-262. 10.3343/alm.2019.39.3.252.
Diagnostic Accuracy of the Risk of Ovarian Malignancy Algorithm in Clinical Practice at a Single Hospital in Korea
- Affiliations
-
- 1Department of Laboratory Medicine, Bucheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 2Department of Obstetrics and Gynecology, Bucheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. leehn@catholic.ac.kr
- KMID: 2431602
- DOI: http://doi.org/10.3343/alm.2019.39.3.252
Abstract
- BACKGROUND
The risk of ovarian malignancy algorithm (ROMA) is used for assessing ovarian cancer risk in women with a pelvic mass. Its diagnostic accuracy is variable. We investigated whether the clinically acceptable minimal sensitivity of >80.0% could be obtained with the suggested cutoff of 7.4%/25.3% for pre/postmenopausal women and with adjusted cutoffs set to a specificity of ≥75.0% or a sensitivity of 95.0%, in a hospital with a lower ovarian cancer (OC) prevalence than previously reported.
METHODS
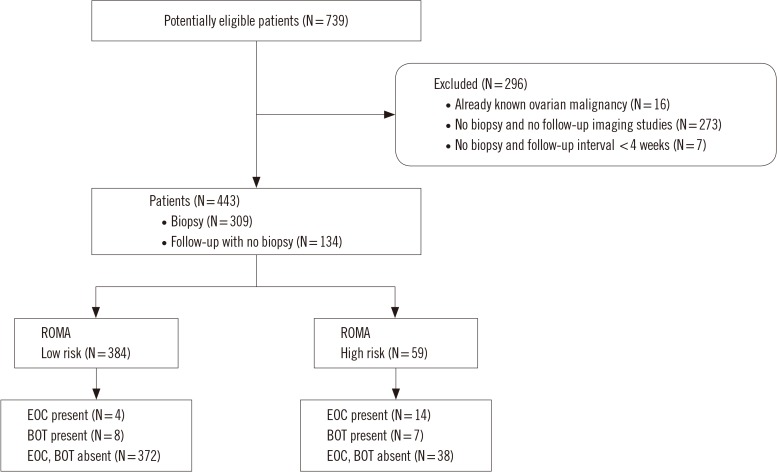
ROMA scores were calculated from measurements of human epididymis protein 4 and cancer antigen 125 in blood specimens from 443 patients with a pelvic mass. The ROMA-based risk group was compared against biopsy (N=309) or clinical follow-up with imaging (N=134) results. The ROMA sensitivity and specificity for predicting epithelial OC (EOC) and borderline ovarian tumor (BOT) were calculated for the suggested and adjusted cutoff values.
RESULTS
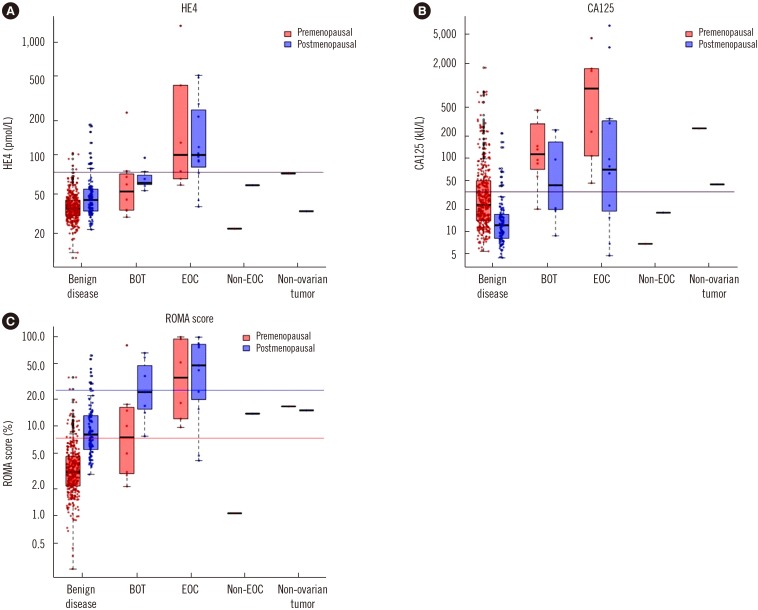
When targeting BOT and EOC, the prevalence was 7.4% and sensitivity and specificity at the suggested cutoff were 63.6% and 90.7%, respectively. Sensitivity was 81.8% at the 4.65%/13.71% cutoff set to a specificity of 75.0%. When targeting only EOC, the prevalence was 4.1% and sensitivity and specificity at the suggested cutoff were 77.8% and 89.4%, respectively. Sensitivity was 88.9% at the 4.78%/14.35% cutoff set to a specificity of 75.0%.
CONCLUSIONS
The sensitivity of ROMA was lower than expected when using the suggested cutoff. When using the adjusted cutoff, its sensitivity reached 80.0%.
Keyword
MeSH Terms
Figure
Reference
-
1. Moore RG, Brown AK, Miller MC, Skates S, Allard WJ, Verch T, et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. 2008; 108:402–408. PMID: 18061248.2. Moore RG, McMeekin DS, Brown AK, DiSilvestro P, Miller MC, Allard WJ, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009; 112:40–46. PMID: 18851871.3. Moore RG, Jabre-Raughley M, Brown AK, Robison KM, Miller MC, Allard WJ, et al. Comparison of a novel multiple marker assay vs the Risk of Malignancy Index for the prediction of epithelial ovarian cancer in patients with a pelvic mass. Am J Obstet Gynecol. 2010; 203:228.e1–228.e6. PMID: 20471625.4. Moore RG, Miller MC, Disilvestro P, Landrum LM, Gajewski W, Ball JJ, et al. Evaluation of the diagnostic accuracy of the risk of ovarian malignancy algorithm in women with a pelvic mass. Obstet Gynecol. 2011; 118:280–288. PMID: 21775843.5. Jia LT, Zhang YC, Li J, Tian Y, Li JF. The role of human epididymis protein 4 in the diagnosis of epithelial ovarian cancer. Clin Transl Oncol. 2016; 18:233–239. PMID: 26220095.6. Dayyani F, Uhlig S, Colson B, Simon K, Rolny V, Morgenstern D, et al. Diagnostic performance of Risk of Ovarian Malignancy Algorithm against CA125 and HE4 in connection with ovarian cancer: a meta-analysis. Int J Gynecol Cancer. 2016; 26:1586–1593. PMID: 27540691.7. Farzaneh F, Honarvar Z, Yaraghi M, Yaseri M, Arab M, Hosseini M, et al. Preoperative evaluation of risk of ovarian malignancy algorithm index in prediction of malignancy of adnexal masses. Iran Red Crescent Med J. 2014; 16:e17185. PMID: 25068046.8. Chan KK, Chen CA, Nam JH, Ochiai K, Wilailak S, Choon AT, et al. The use of HE4 in the prediction of ovarian cancer in Asian women with a pelvic mass. Gynecol Oncol. 2013; 128:239–244. PMID: 23063998.9. Winarto H, Laihad BJ, Nuranna L. Modification of cutoff values for HE4, CA125, the Risk of Malignancy Index, and the Risk of Malignancy Algorithm for ovarian cancer detection in Jakarta, Indonesia. Asian Pac J Cancer Prev. 2014; 15:1949–1953. PMID: 24716917.10. Chen X, Zhou H, Chen R, He J, Wang Y, Huang L, et al. Development of a multimarker assay for differential diagnosis of benign and malignant pelvic masses. Clin Chim Acta. 2015; 440:57–63. PMID: 25447698.11. Xu Y, Zhong R, He J, Ding R, Lin H, Deng Y, et al. Modification of cut-off values for HE4, CA125 and the ROMA algorithm for early-stage epithelial ovarian cancer detection: results from 1021 cases in South China. Clin Biochem. 2016; 49:32–40. PMID: 26285075.12. Karlsen MA, Høgdall EV, Christensen IJ, Borgfeldt C, Kalapotharakos G, Zdrazilova-Dubska L, et al. A novel diagnostic index combining HE4, CA125 and age may improve triage of women with suspected ovarian cancer–an international multicenter study in women with an ovarian mass. Gynecol Oncol. 2015; 138:640–646. PMID: 26086566.13. Ruggeri G, Bandiera E, Zanotti L, Belloli S, Ravaggi A, Romani C, et al. HE4 and epithelial ovarian cancer: comparison and clinical evaluation of two immunoassays and a combination algorithm. Clin Chim Acta. 2011; 412:1447–1453. PMID: 21557935.14. Lenhard M, Stieber P, Hertlein L, Kirschenhofer A, Fürst S, Mayr D, et al. The diagnostic accuracy of two human epididymis protein 4 (HE4) testing systems in combination with CA125 in the differential diagnosis of ovarian masses. Clin Chem Lab Med. 2011; 49:2081–2088. PMID: 21923475.15. Karlsen MA, Sandhu N, Høgdall C, Christensen IJ, Nedergaard L, Lundvall L, et al. Evaluation of HE4, CA125, risk of ovarian malignancy algorithm (ROMA) and risk of malignancy index (RMI) as diagnostic tools of epithelial ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2012; 127:379–383. PMID: 22835718.16. Sandri MT, Bottari F, Franchi D, Boveri S, Candiani M, Ronzoni S, et al. Comparison of HE4, CA125 and ROMA algorithm in women with a pelvic mass: correlation with pathological outcome. Gynecol Oncol. 2013; 128:233–238. PMID: 23200911.17. Romagnolo C, Leon AE, Fabricio ASC, Taborelli M, Polesel J, Del Pup L, et al. HE4, CA125 and risk of ovarian malignancy algorithm (ROMA) as diagnostic tools for ovarian cancer in patients with a pelvic mass: an Italian multicenter study. Gynecol Oncol. 2016; 141:303–311. PMID: 26801941.18. U.S. Food and Drug Administration. K103358 ROMA (HE4 EIA + Architect CA 125 II) 510(k) Substantial equivalence determination decision summary. Updated on Oct 2018. https://www.accessdata.fda.gov/cdrh_docs/reviews/K103358.pdf.19. Kim YM, Whang DH, Park J, Kim SH, Lee SW, Park HA, et al. Evaluation of the accuracy of serum human epididymis protein 4 in combination with CA125 for detecting ovarian cancer: a prospective case-control study in a Korean population. Clin Chem Lab Med. 2011; 49:527–534. PMID: 21320028.20. Granato T, Porpora MG, Longo F, Angeloni A, Manganaro L, Anastasi E. HE4 in the differential diagnosis of ovarian masses. Clin Chim Acta. 2015; 446:147–155. PMID: 25892674.21. Irwig LM, Bossuyt PMM, Glasziou PP, Gatsonis C, Lijmer JG. Designing studies to ensure that estimates of test accuracy will travel. In : Knottnerus JA, Buntinx F, editors. The evidence base of clinical diagnosis: theory and methods of diagnostic research. 2nd ed. West Sussex, UK: Blackwell Publishing Ltd. BMJ Books;2009. p. 96–117.22. Haynes RB, You JJ. The architecture of diagnostic research. In : Knottnerus JA, Buntinx F, editors. The evidence base of clinical diagnosis: theory and methods of diagnostic research. 2nd ed. West Sussex, UK: Blackwell Publishing Ltd. BMJ Books;2009. p. 20–41.23. Knottnerus JA, Muris JW. Assessment of the accuracy of diagnostic tests: the cross-sectional study. In : Knottnerus JA, Buntinx F, editors. The evidence base of clinical diagnosis: theory and methods of diagnostic research. 2nd ed. West Sussex, UK: Blackwell Publishing Ltd. BMJ Books;2009. p. 42–62.24. López-Ratón M, Rodríguez-Álvarez MX, Cadarso-Suárez C, Gude-Sampedro F. Optimal Cutpoints: an R package for selecting optimal cut points in diagnostic tests. J Stat Softw. 2014; 61:1–36.25. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing;Updated on Sep 2018. https://www.R-project.org.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Human Epididymis Protein 4 as a Diagnostic Marker of Ovarian Cancer and Its Reference Interval in Korean Population
- Comparison of four malignancy risk indices in the detection of malignant ovarian masses
- The power of the Risk of Ovarian Malignancy Algorithm considering menopausal status: a comparison with CA 125 and HE4
- The Accuracy of Frozen Section in the Diagnosis of Ovarian Tumors
- Analysis of falsely elevated risk of ovarian malignancy algorithm in women with ovarian endometrioma