Ann Lab Med.
2018 Nov;38(6):530-537. 10.3343/alm.2018.38.6.530.
Clinical Utility and Cross-Reactivity of Insulin and C-Peptide Assays by the Lumipulse G1200 System
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. nayadoo@hanmail.net
- 2Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2429118
- DOI: http://doi.org/10.3343/alm.2018.38.6.530
Abstract
- BACKGROUND
Measurement of insulin and C-peptide concentrations is important for deciding whether insulin treatment is required in diabetic patients. We aimed to investigate the analytical performance of insulin and C-peptide assays using the Lumipulse G1200 system (Fujirebio Inc., Tokyo, Japan).
METHODS
We examined the precision, linearity, and cross-reactivity of insulin and C-peptide using five insulin analogues and purified proinsulin. A method comparison was conducted between the Lumipulse G1200 and Roche E170 (Roche Diagnostics, Mannheim, Germany) systems in 200 diabetic patients on insulin treatment. Reference intervals for insulin and C-peptide concentrations were determined in 279 healthy individuals.
RESULTS
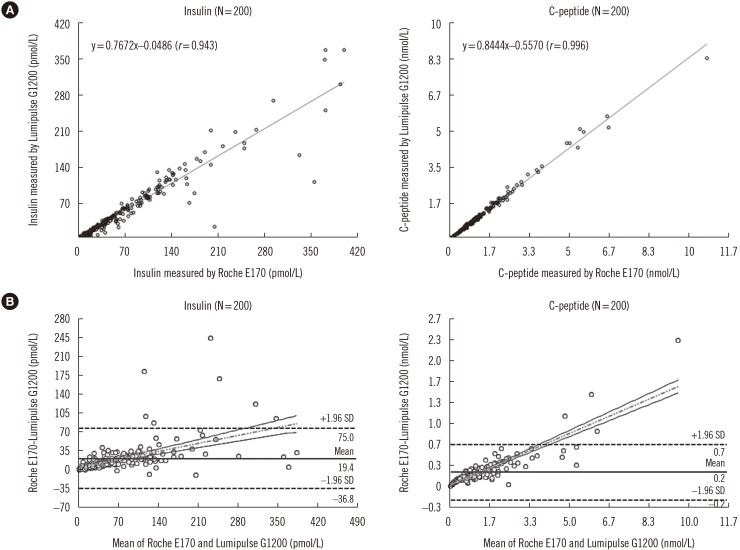
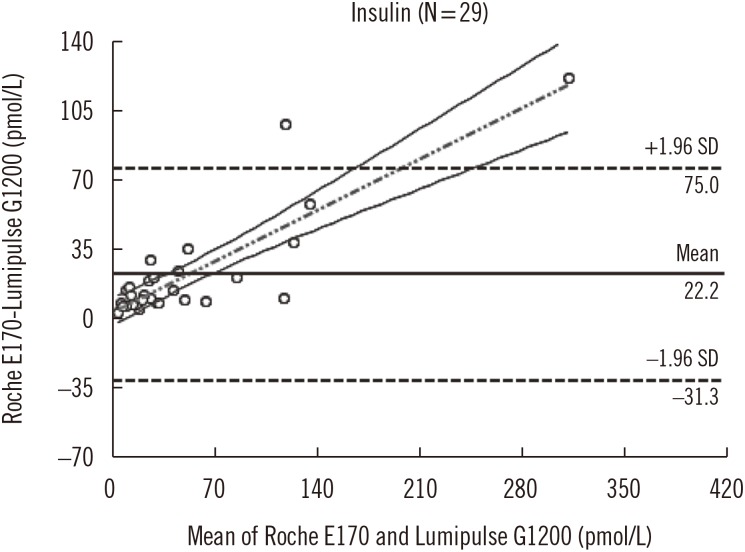
For insulin and C-peptide assays, within-laboratory precision (% CV) was 3.78-4.14 and 2.89-3.35%, respectively. The linearity of the insulin assay in the range of 0-2,778 pmol/L was R2=0.9997, and that of the C-peptide assay in the range of 0-10 nmol/L was R2=0.9996. The correlation coefficient (r) between the Roche E170 and Lumipulse G1200 results was 0.943 (P < 0.001) for insulin and 0.996 (P < 0.001) for C-peptide. The mean differences in insulin and C-peptide between Lumipulse G1200 and the Roche E170 were 19.4 pmol/L and 0.2 nmol/L, respectively. None of the insulin analogues or proinsulin showed significant cross-reactivity with the Lumipulse G1200. Reference intervals of insulin and C-peptide were 7.64-70.14 pmol/L and 0.17-0.85 nmol/L, respectively.
CONCLUSIONS
Insulin and C-peptide tests on the Lumipulse G1200 show adequate analytical performance and are expected to be acceptable for use in clinical areas.
Keyword
Figure
Cited by 1 articles
-
Reference Standards for C-Peptide in Korean Population: A Korean Endocrine Hormone Reference Standard Data Center Study
Jooyoung Cho, Ho-Chan Cho, Ohk-Hyun Ryu, Hyo-Jeong Kim, Chang Geun Kim, Young Ran Yun, Choon Hee Chung
Endocrinol Metab. 2024;39(3):489-499. doi: 10.3803/EnM.2023.1888.
Reference
-
1. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014; 103:137–149. PMID: 24630390.2. Kim SY. It's still not too late to make a change: current status of glycemic control in Korea. Diabetes Metab J. 2014; 38:194–196. PMID: 25003072.3. Song SO, Song YD, Nam JY, Park KH, Yoon JH, Son KM, et al. Epidemiology of type 1 diabetes mellitus in Korea through an investigation of the national registration project of type 1 diabetes for the reimbursement of glucometer strips with additional analyses using claims data. Diabetes Metab J. 2016; 40:35–45. PMID: 26912154.4. American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2016; 39(S1):S13–S22. PMID: 26696675.5. Jones AG, Hattersley AT. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet Med. 2013; 30:803–817. PMID: 23413806.6. Oram RA, Patel K, Hill A, Shields B, McDonald TJ, Jones A, et al. A type 1 diabetes genetic risk score can aid discrimination between type 1 and type 2 diabetes in young adults. Diabetes Care. 2016; 39:337–344. PMID: 26577414.7. Clark PM. Assays for insulin, proinsulin(s) and C-peptide. Ann Clin Biochem. 1999; 36(Pt 5):541–564. PMID: 10505204.8. Marcovina S, Bowsher RR, Miller WG, Staten M, Myers G, Caudill SP, et al. Standardization of insulin immunoassays: report of the American Diabetes Association Workgroup. Clin Chem. 2007; 53:711–716. PMID: 17272483.9. Jones AG, Besser RE, Shields BM, McDonald TJ, Hope SV, Knight BA, et al. Assessment of endogenous insulin secretion in insulin treated diabetes predicts postprandial glucose and treatment response to prandial insulin. BMC Endocr Disord. 2012; 12:6. PMID: 22681724.10. Dayaldasani A, Rodriguez Espinosa M, Ocon Sanchez P, Perez Valero V. Cross-reactivity of insulin analogues with three insulin assays. Ann Clin Biochem. 2015; 52:312–318. PMID: 25172526.11. Heald A, Bhattacharya B, Cooper H, Ullah A, McCulloch A, Smellie S, et al. Most commercial insulin assays fail to detect recombinant insulin analogues. Ann Clin Biochem. 2006; 43:306–308. PMID: 16824282.12. Sapin R. Insulin assays: previously known and new analytical features. Clin Lab. 2003; 49:113–121. PMID: 12705692.13. Nakahara Y, Kiya A, Uchida T, Uno J, Maeda A. Comparative study of three blood insulin measuring reagents with different measurement principles. Okayama J Med Technol. 2015; 51:1–6.14. Nalbantoglu Elmas O, Demir K, Soylu N, Celik N, Ozkan B. Importance of insulin immunoassays in the diagnosis of factitious hypoglycemia. J Clin Res Pediatr Endocrinol. 2014; 6:258–261. PMID: 25541899.15. CLSI. Evaluation of precision of quantitative measurement procedures; approved guideline. 3rd ed. Wayne, PA: Clinical and Laboratory Standards Institute;2014. CLSI EP05-A3.16. CLSI. Evaluation of the linearity of quantitative measurement procedures: A statistical approach; approved guideline. CLSI EP06-A. Wayne, PA: Clinical and Laboratory Standards Institute;2003.17. CLSI. Method comparison and bias estimation using patient samples; approved guideline. 2nd ed. (Interim Revision). Wayne, PA: Clinical and Laboratory Standards Institute;2013. CLSI EP09-A2-IR.18. CLSI. Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline. 3rd ed. Wayne, PA: Clinical and Laboratory Standards Institute;2010. CLSI C28-A3c.19. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986; 1:307–310. PMID: 2868172.20. Ozturk O. Using biological variation data for reference change values in clinical laboratories. Biochem Anal Biochem. 2012; 1:e106.21. Ochocinska A, Snitko R, Czekuc-Kryskiewicz E, Kepka A, Szalecki M, Janas RM. Evaluation of the immunoradiometric and electrochemiluminescence method for the measurement of serum insulin in children. J Immunoassay Immunochem. 2016; 37:243–250. PMID: 26962675.22. Loh TP, Sutanto S, Khoo CM. Comparison of three routine insulin immunoassays: implications for assessment of insulin sensitivity and response. Clin Chem Lab Med. 2017; 55:e72–e75. PMID: 27665422.23. Chevenne D, Trivin F, Porquet D. Insulin assays and reference values. Diabetes Metab. 1999; 25:459–476. PMID: 10633871.24. Owen WE, Roberts WL. Cross-reactivity of three recombinant insulin analogs with five commercial insulin immunoassays. Clin Chem. 2004; 50:257–259. PMID: 14709671.25. Parfitt C, Church D, Armston A, Couchman L, Evans C, Wark G, et al. Commercial insulin immunoassays fail to detect commonly prescribed insulin analogues. Clin Biochem. 2015; 48:1354–1357. PMID: 26171976.26. Hirsch IB. Insulin analogues. N Engl J Med. 2005; 352:174–183. PMID: 15647580.27. Vajo Z, Duckworth WC. Genetically engineered insulin analogs: diabetes in the new millenium. Pharmacol Rev. 2000; 52:1–9. PMID: 10699152.28. Kim S, Yun YM, Hur M, Moon HW. Unusually elevated serum insulin level in a diabetic patient during recombinant insulin therapy. Lab Med Online. 2013; 3:56–59.29. Kim S, Yun YM, Hur M, Moon HW, Kim JQ. The effects of anti-insulin antibodies and cross-reactivity with human recombinant insulin analogues in the E170 insulin immunometric assay. Korean J Lab Med. 2011; 31:22–29. PMID: 21239867.30. Sapin R, Le Galudec V, Gasser F, Pinget M, Grucker D. Elecsys insulin assay: free insulin determination and the absence of cross-reactivity with insulin lispro. Clin Chem. 2001; 47:602–605. PMID: 11238323.31. Moriyama M, Hayashi N, Ohyabu C, Mukai M, Kawano S, Kumagai S. Performance evaluation and cross-reactivity from insulin analogs with the ARCHITECT insulin assay. Clin Chem. 2006; 52:1423–1426. PMID: 16690737.32. Glenn C, Armston A. Cross-reactivity of 12 recombinant insulin preparations in the Beckman Unicel DxI 800 insulin assay. Ann Clin Biochem. 2010; 47:264–266. PMID: 20392753.33. Kalathil S, Napier C, Pattman SJ, Wark G, Abouglila K, James RA. Variable characteristics with insulin assays. Pract Diabetes. 2013; 30:118–120.34. Vieira JG, Tachibana TT, Ferrer CM, Reis AF. Cross-reactivity of new insulin analogs in insulin assays. Arq Bras Endocrinol Metabol. 2007; 51:504–505. PMID: 17546253.35. Agin A, Jeandidier N, Gasser F, Grucker D, Sapin R. Glargine blood biotransformation: in vitro appraisal with human insulin immunoassay. Diabetes Metab. 2007; 33:205–212. PMID: 17360218.36. Song D, Davidson J. Cross-reactivity of Actrapid and three insulin analogues in the Abbott IMx insulin immunoassay. Ann Clin Biochem. 2007; 44:197–198. PMID: 17362590.37. Neal JM, Han W. Insulin immunoassays in the detection of insulin analogues in factitious hypoglycemia. Endocr Pract. 2008; 14:1006–1010. PMID: 19095600.38. Palmer JP, Fleming GA, Greenbaum CJ, Herold KC, Jansa LD, Kolb H, et al. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21-22 October 2001. Diabetes. 2004; 53:250–264. PMID: 14693724.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of the Performance of Lumipulse G1200 for Tumor Marker Assays
- The Effects of Anti-insulin Antibodies and Cross-reactivity with Human Recombinant Insulin Analogues in the E170 Insulin Immunometric Assay
- Improvement of Insulin Secretoruy Capacity According to Insulin Therapy in Non-Insulin Depentent Diabetes Mellitus
- HLA Typing, Islet Cell Antibody and C-Peptide of Insulin Dependent Diabetes Mellitus in Children
- Ensulin Autoimmune Syndrome in a Patient with Methimazole-Treated Graves' Disease: A Case report