Allergy Asthma Immunol Res.
2018 Jan;10(1):52-61. 10.4168/aair.2018.10.1.52.
β₂-Adrenoceptor Blockade Deteriorates Systemic Anaphylaxis by Enhancing Hyperpermeability in Anesthetized Mice
- Affiliations
-
- 1Department of Physiology II, Kanazawa Medical University, Uchinada, Japan. shibamo@kanazawa-med.ac.jp
- 2Department of Infectious Disease, Shengjing Hospital of China Medical University, Shenyang, China.
- 3Department of Colorectal and Hernia Surgery, The Fourth Affiliated Hospital of China Medical University, Shenyang, China.
- KMID: 2428850
- DOI: http://doi.org/10.4168/aair.2018.10.1.52
Abstract
- PURPOSE
Patients treated with propranolol, a nonselective β-adrenoceptor antagonist, develop severe anaphylaxis, but the mechanism remains unknown. We determined effects of βâ‚- and β₂-adrenoceptor antagonists on the anaphylaxis-induced increase in vascular permeability in mice.
METHODS
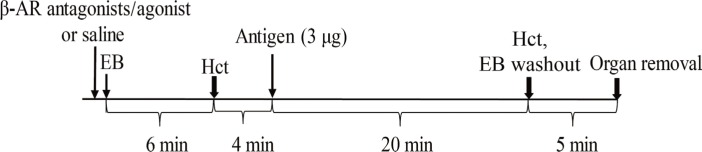
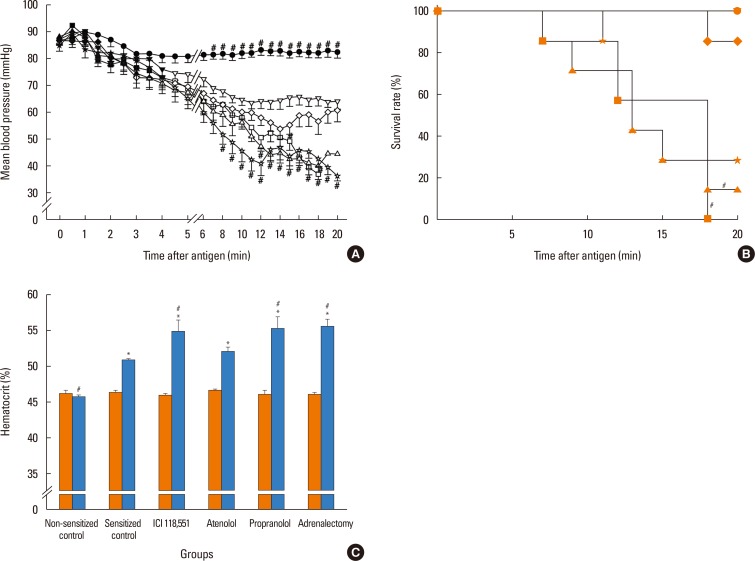
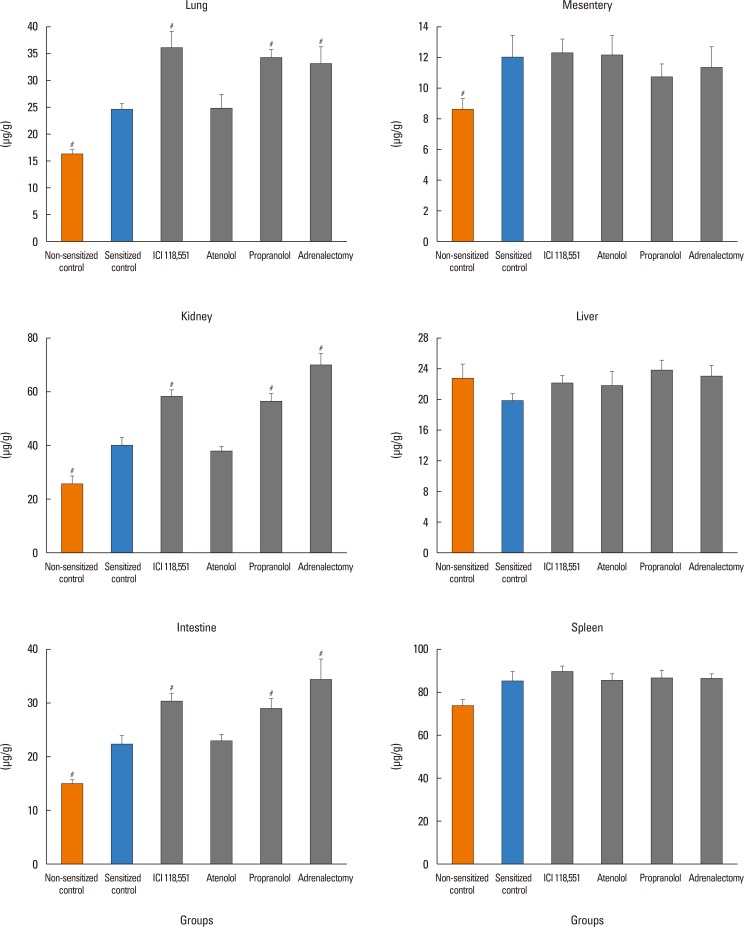
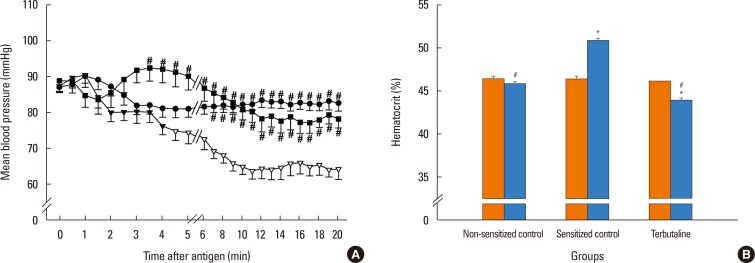
In anesthetized ovalbumin-sensitized C57BL mice, mean arterial blood pressure (MBP) was measured, and Evans blue dye extravasation and hematocrit (Hct) were assessed at 20 minutes after antigen injection. The following pretreatment groups (n=7/group) were studied: (1) sensitized control (non-pretreatment), (2) propranolol, (3) the selective β₂-adrenoceptor antagonist ICI 118,551, (4) the selective βâ‚-adrenoceptor antagonist atenolol, (5) adrenalectomy, (6) the selective β₂-adrenoceptor agonist terbutaline, and (7) non-sensitized groups.
RESULTS
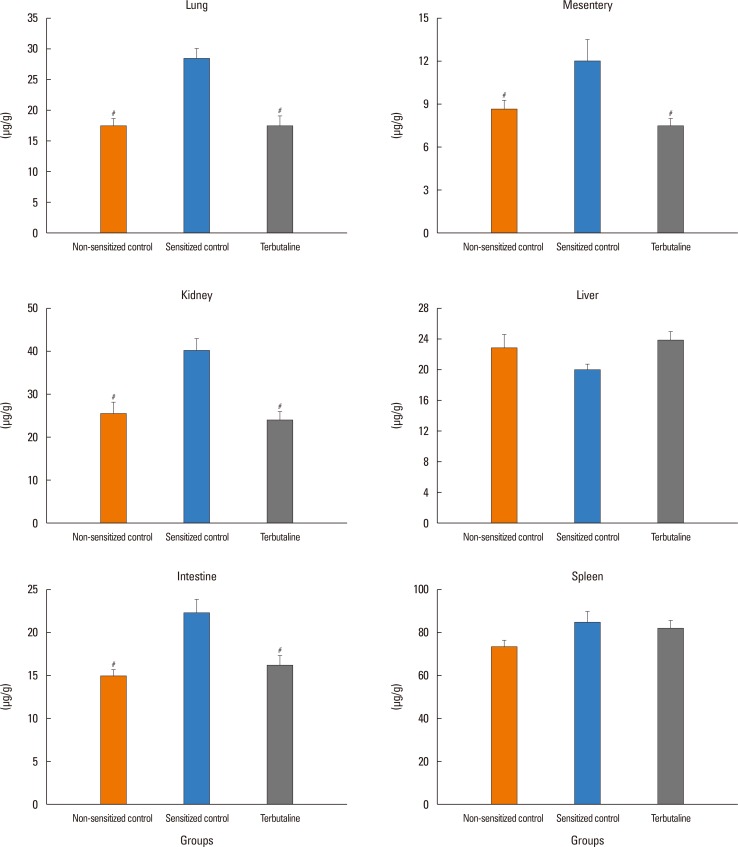
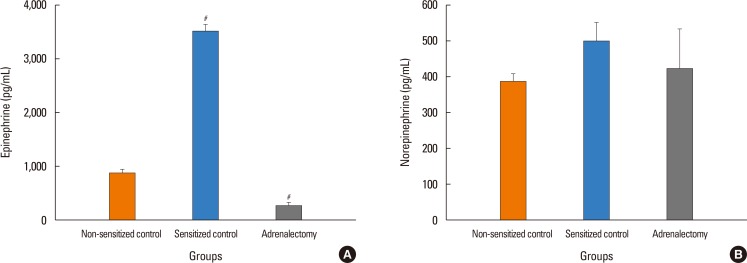
The antigen injection decreased MBP, and increased Hct and vascular permeability in the kidney, lung, mesentery, and intestine, but not in the liver or spleen. Pretreatment with ICI 118,551, propranolol and adrenalectomy, but not atenolol, reduced the survival rate and augmented the increases in Hct and vascular permeability in the kidney, intestine, and lung as compared with the sensitized control group. Pretreatment with terbutaline abolished the antigen-induced alterations. Plasma epinephrine levels were increased significantly in the sensitize control mice.
CONCLUSIONS
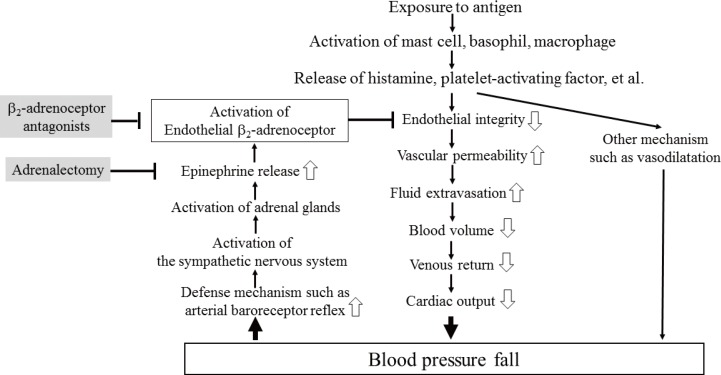
Blockade of β₂-adrenoceptor can deteriorate systemic anaphylaxis by augmenting hyperpermeability-induced increase in plasma extravasation by inhibiting beneficial effects of epinephrine released from the adrenal glands in anesthetized mice.
Keyword
MeSH Terms
Figure
Reference
-
1. Sampson HA, Muñoz-Furlong A, Bock SA, Schmitt C, Bass R, Chowdhury BA, et al. Symposium on the definition and management of anaphylaxis: summary report. J Allergy Clin Immunol. 2005; 115:584–591. PMID: 15753908.
Article2. Fisher MM. Clinical observations on the pathophysiology and treatment of anaphylactic cardiovascular collapse. Anaesth Intensive Care. 1986; 14:17–21. PMID: 2869715.
Article3. Bergman RK, Munoz J. Circulatory collapse in anaphylaxis and histamine toxicity in mice. J Immunol. 1965; 95:1–8. PMID: 14328697.4. Cui S, Shibamoto T, Zhang W, Takano H, Kurata Y. Venous resistance increases during rat anaphylactic shock. Shock. 2008; 29:733–739. PMID: 17998891.
Article5. Leng W, Chang K, Williamson JR, Jakschik BA. Increased regional vascular albumin permeation in the rat during anaphylaxis. J Immunol. 1989; 142:1982–1985. PMID: 2921522.6. Miyasaka CK, Mendonça JR, Silva ZL, de Sousa JA, Tavares de Lima W, Curi R. Modulation of hypersensitivity reaction by lipids given orally. Gen Pharmacol. 1999; 32:597–602. PMID: 10382863.
Article7. Jancar S, Sirois MG, Carrier J, Braquet P, Sirois P. PAF induces rat plasma extravasation and releases eicosanoids during anaphylaxis. Inflammation. 1991; 15:347–354. PMID: 1757122.
Article8. Lang DM. Anaphylactoid and anaphylactic reactions. Hazards of beta-blockers. Drug Saf. 1995; 12:299–304. PMID: 7669259.9. Mueller UR. Cardiovascular disease and anaphylaxis. Curr Opin Allergy Clin Immunol. 2007; 7:337–341. PMID: 17620826.
Article10. Zhang W, Shibamoto T, Kuda Y, Ohmukai C, Kurata Y. Pulmonary vasoconstrictive and bronchoconstrictive responses to anaphylaxis are weakened via β2-adrenoceptor activation by endogenous epinephrine in anesthetized rats. Anesthesiology. 2011; 114:614–623. PMID: 21307766.11. Ding Z, Jiang M, Li S, Zhang Y. Vascular barrier-enhancing effect of an endogenous beta-adrenergic agonist. Inflammation. 1995; 19:1–8. PMID: 7705881.12. Liu W, Takano H, Shibamoto T, Cui S, Zhao ZS, Zhang W, et al. Involvement of splanchnic vascular bed in anaphylactic hypotension in anesthetized BALB/c mice. Am J Physiol Regul Integr Comp Physiol. 2007; 293:R1947–R1953. PMID: 17715178.
Article13. Rajab M, Jin H, Welzig CM, Albano A, Aronovitz M, Zhang Y, et al. Increased inducibility of ventricular tachycardia and decreased heart rate variability in a mouse model for type 1 diabetes: effect of pravastatin. Am J Physiol Heart Circ Physiol. 2013; 305:H1807–H1816. PMID: 24163078.
Article14. Rose RA, Kabir MG, Backx PH. Altered heart rate and sinoatrial node function in mice lacking the cAMP regulator phosphoinositide 3-kinase-gamma. Circ Res. 2007; 101:1274–1282. PMID: 17975110.15. Shinomiya S, Shibamoto T, Kurata Y, Kuda Y, Zhang W, Tanida M, et al. Nitric oxide and β2-adrenoceptor activation attenuate pulmonary vasoconstriction during anaphylactic hypotension in anesthetized BALB/c mice. Exp Lung Res. 2013; 39:119–129. PMID: 23442108.
Article16. Chen LT. Microcirculation of the spleen: and open or closed circulation? Science. 1978; 201:157–159. PMID: 663644.17. Naito M, Wisse E. Filtration effect of endothelial fenestrations on chylomicron transport in neonatal rat liver sinusoids. Cell Tissue Res. 1978; 190:371–382. PMID: 567529.
Article18. Finkelman FD, Rothenberg ME, Brandt EB, Morris SC, Strait RT. Molecular mechanisms of anaphylaxis: lessons from studies with murine models. J Allergy Clin Immunol. 2005; 115:449–457. PMID: 15753886.
Article19. Wang M, Shibamoto T, Tanida M, Kuda Y, Kurata Y. Mouse anaphylactic shock is caused by reduced cardiac output, but not by systemic vasodilatation or pulmonary vasoconstriction, via PAF and histamine. Life Sci. 2014; 116:98–105. PMID: 25252221.
Article20. Knezevic II, Predescu SA, Neamu RF, Gorovoy MS, Knezevic NM, Easington C, et al. Tiam1 and Rac1 are required for platelet-activating factor-induced endothelial junctional disassembly and increase in vascular permeability. J Biol Chem. 2009; 284:5381–5394. PMID: 19095647.
Article21. Miles AA, Miles EM. Vascular reactions to histamine, histamine-liberator and leukotaxine in the skin of guinea-pigs. J Physiol. 1952; 118:228–257. PMID: 13000707.
Article22. Harris ES, Nelson WJ. VE-cadherin: at the front, center, and sides of endothelial cell organization and function. Curr Opin Cell Biol. 2010; 22:651–658. PMID: 20708398.
Article23. Vestweber D, Winderlich M, Cagna G, Nottebaum AF. Cell adhesion dynamics at endothelial junctions: VE-cadherin as a major player. Trends Cell Biol. 2009; 19:8–15. PMID: 19010680.
Article24. Birukova AA, Shah AS, Tian Y, Gawlak G, Sarich N, Birukov KG. Selective role of vinculin in contractile mechanisms of endothelial permeability. Am J Respir Cell Mol Biol. 2016; 55:476–486. PMID: 27115795.
Article25. Adderley SP, Zhang XE, Breslin JW. Involvement of the H1 histamine receptor, p38 MAP kinase, myosin light chains kinase, and Rho/ROCK in histamine-induced endothelial barrier dysfunction. Microcirculation. 2015; 22:237–248. PMID: 25582918.
Article26. Mikelis CM, Simaan M, Ando K, Fukuhara S, Sakurai A, Amornphimoltham P, et al. RhoA and ROCK mediate histamine-induced vascular leakage and anaphylactic shock. Nat Commun. 2015; 6:6725. PMID: 25857352.
Article27. Andriopoulou P, Navarro P, Zanetti A, Lampugnani MG, Dejana E. Histamine induces tyrosine phosphorylation of endothelial cell-to-cell adherens junctions. Arterioscler Thromb Vasc Biol. 1999; 19:2286–2297. PMID: 10521356.
Article28. Unwalla HJ, Horvath G, Roth FD, Conner GE, Salathe M. Albuterol modulates its own transepithelial flux via changes in paracellular permeability. Am J Respir Cell Mol Biol. 2012; 46:551–558. PMID: 22162907.
Article29. Spindler V, Waschke J. Beta-adrenergic stimulation contributes to maintenance of endothelial barrier functions under baseline conditions. Microcirculation. 2011; 18:118–127. PMID: 21166930.
Article30. Birukova AA, Alekseeva E, Mikaelyan A, Birukov KG. HGF attenuates thrombin-induced endothelial permeability by Tiam1-mediated activation of the Rac pathway and by Tiam1/Rac-dependent inhibition of the Rho pathway. FASEB J. 2007; 21:2776–2786. PMID: 17428964.31. Bindewald K, Gündüz D, Härtel F, Peters SC, Rodewald C, Nau S, et al. Opposite effect of cAMP signaling in endothelial barriers of different origin. Am J Physiol Cell Physiol. 2004; 287:C1246–C1255. PMID: 15475517.
Article32. Li AQ, Zhao L, Zhou TF, Zhang MQ, Qin XM. Exendin-4 promotes endothelial barrier enhancement via PKA- and Epac1-dependent Rac1 activation. Am J Physiol Cell Physiol. 2015; 308:C164–C175. PMID: 25377089.
Article33. Abdelrahman A, Tabrizchi R, Pang CC. Effects of beta 1- and beta 2-adrenoceptor stimulation on hemodynamics in the anesthetized rat. J Cardiovasc Pharmacol. 1990; 15:720–728. PMID: 1692931.34. Hein TW, Zhang C, Wang W, Kuo L. Heterogeneous beta2-adrenoceptor expression and dilation in coronary arterioles across the left ventricular wall. Circulation. 2004; 110:2708–2712. PMID: 15249507.35. Watson DC, Sargianou M, Leivaditis V, Anagnostopoulos C. Beta2-adrenergic activation via administration of atenolol/formoterol combination increases contractility and coronary blood flow in isolated rat hearts. Hellenic J Cardiol. 2013; 54:341–347. PMID: 24100176.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cardiac physiologic regulation of sub-type specific adrenergic receptors in transgenic mice overexpressing βâ‚- and β₂-adrenergic receptors
- On the Site and Mechanism of Action of β₃-Adrenoceptor Agonists in the Bladder
- Cardiac Physiologic Regulation of Sub-type Specific Adrenergic Receptors in Transgenic Mice Overexpressing β1- and β2-Adrenergic Receptors
- Inhibitory effects of ethanol feeding on active systemic anaphylaxis in mice
- A Clinician's Perspective on the Mechanism of β₃-Adrenoceptor Agonists in the Bladder