Korean J Radiol.
2017 Apr;18(2):309-322. 10.3348/kjr.2017.18.2.309.
Ultrasound-Guided Percutaneous Core Needle Biopsy of Abdominal Viscera: Tips to Ensure Safe and Effective Biopsy
- Affiliations
-
- 1Department of Radiology, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju 61469, Korea. kjradsss@dreamwiz.com
- 2Center for Aging and Geriatrics, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju 61469, Korea.
- KMID: 2427943
- DOI: http://doi.org/10.3348/kjr.2017.18.2.309
Abstract
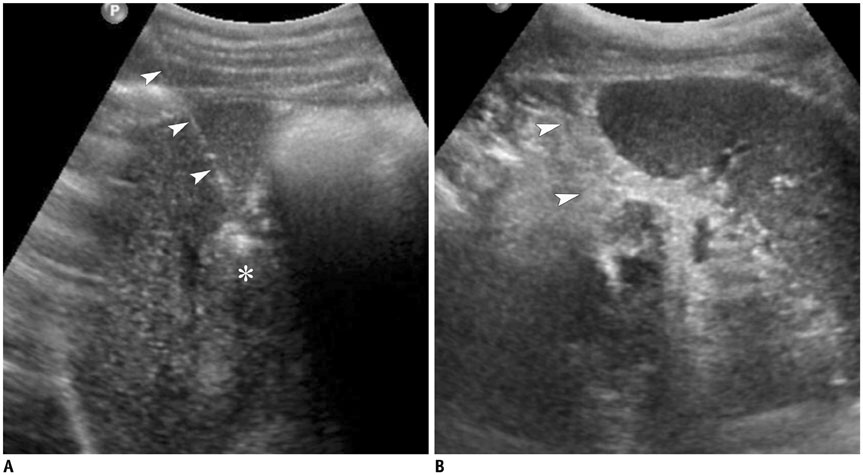
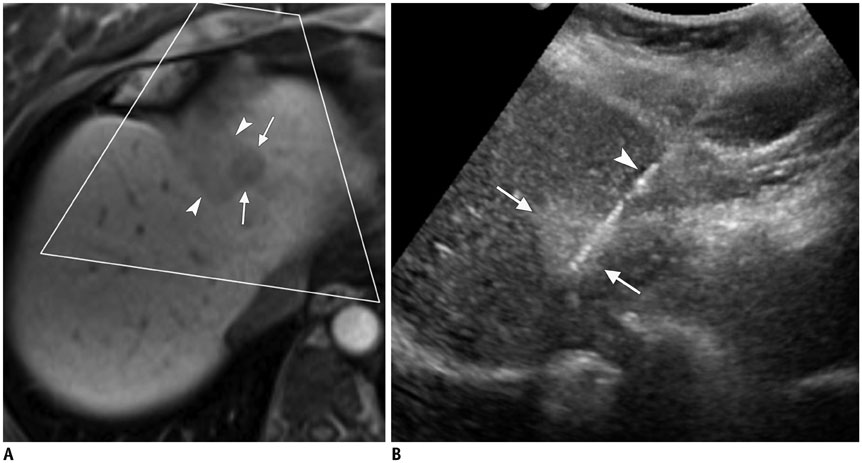
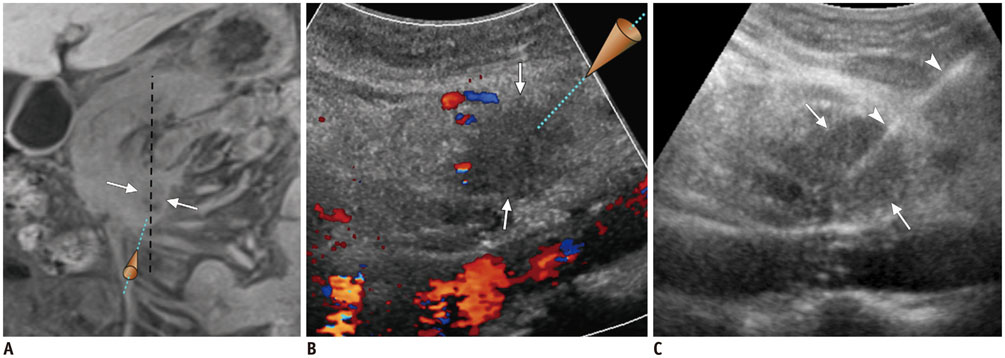
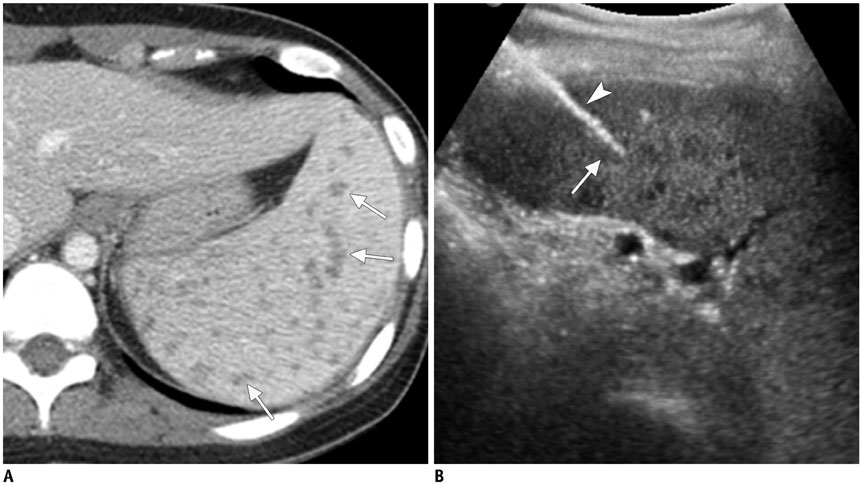
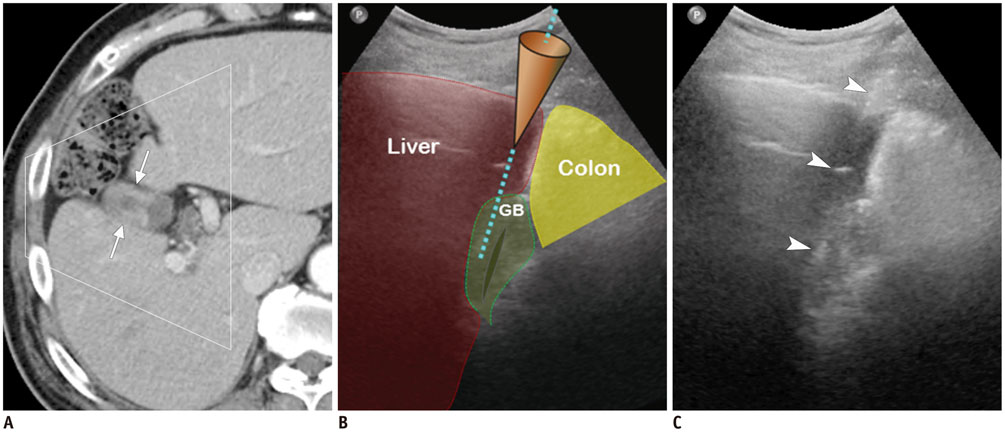
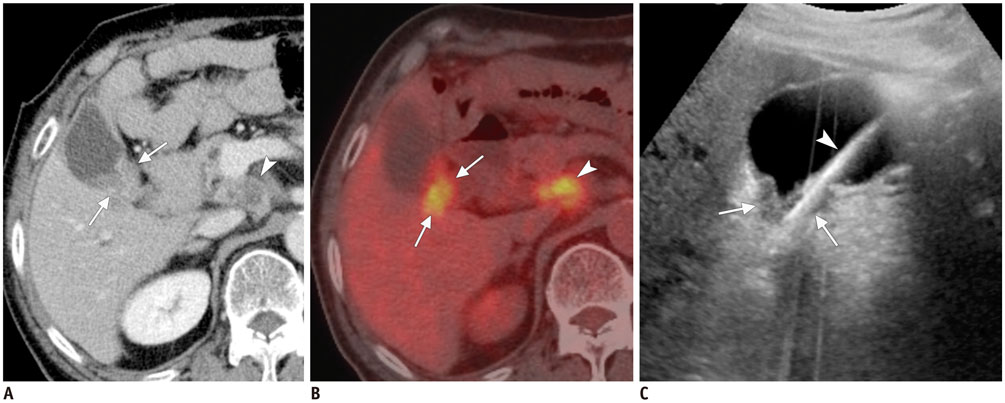
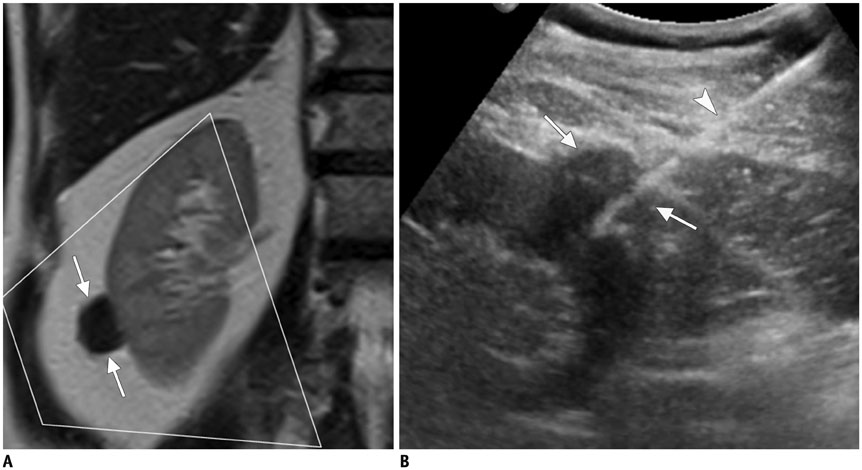
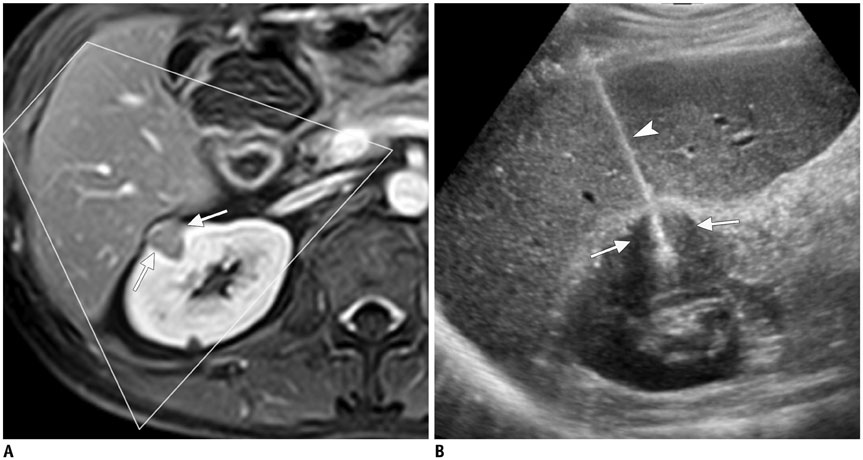
- Ultrasound-guided percutaneous core needle biopsy (USPCB) is used extensively in daily clinical practice for the pathologic confirmation of both focal and diffuse diseases of the abdominal viscera. As a guidance tool, US has a number of clear advantages over computerized tomography or magnetic resonance imaging: fewer false-negative biopsies, lack of ionizing radiation, portability, relatively short procedure time, real-time intra-procedural visualization of the biopsy needle, ability to guide the procedure in almost any anatomic plane, and relatively lower cost. Notably, USPCB is widely used to retrieve tissue specimens in cases of hepatic lesions. However, general radiologists, particularly beginners, find USPCB difficult to perform in abdominal organs other than the liver; indeed, a full understanding of the entire USPCB process and specific considerations for specific abdominal organs is necessary to safely obtain adequate specimens. In this review, we discuss some points and techniques that need to be borne in mind to increase the chances of successful USPCB. We believe that the tips and considerations presented in this review will help radiologists perform USPCB to successfully retrieve target tissue from different organs with minimal complications.
Keyword
MeSH Terms
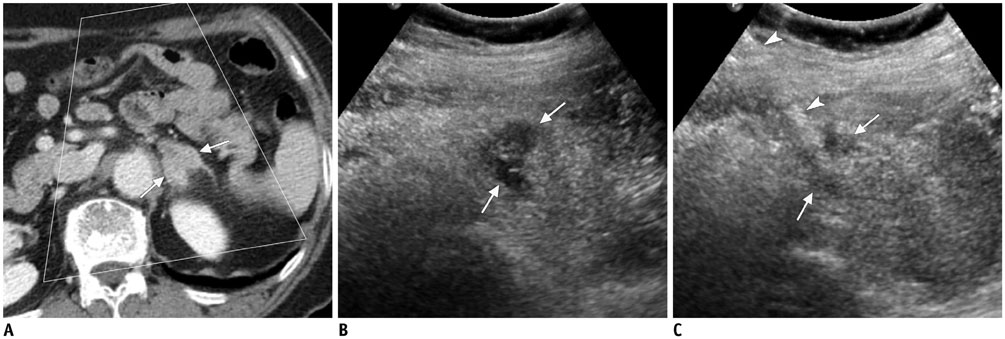
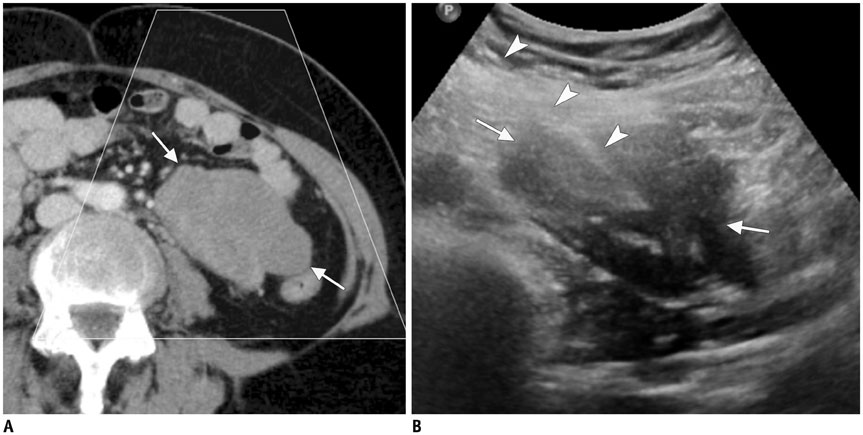
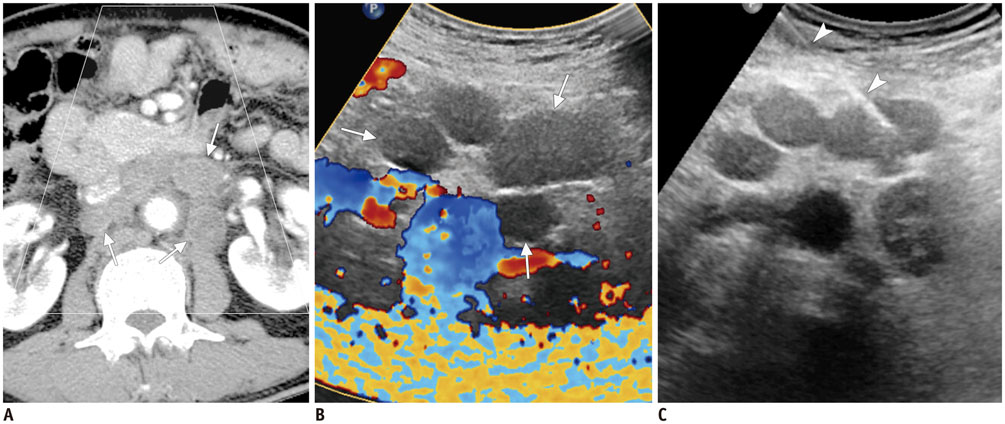
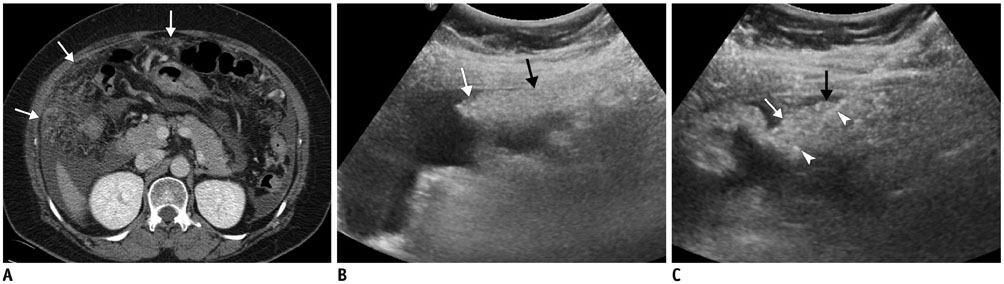
Figure
Reference
-
1. Reading CC. Percutaneous needle biopsy. Abdom Imaging. 1997; 22:311–312.2. Atwell T, Charboneau JW, McGahan J, Reading CC. Ultrasound-guided biopsy of abdomen and pelvis. In : Rumack CM, Wilson SR, Charboneau JW, Levine D, editors. Diagnostic Ultrasound. 4th ed. Philadelphia, PA: Elsevier & Mosby;2011. p. 613–638.3. Jennings PE, Donald JJ, Coral A, Rode J, Lees WR. Ultrasound-guided core biopsy. Lancet. 1989; 1:1369–1371.4. Sheafor DH, Paulson EK, Simmons CM, DeLong DM, Nelson RC. Abdominal percutaneous interventional procedures: comparison of CT and US guidance. Radiology. 1998; 207:705–710.5. Charboneau JW, Reading CC, Welch TJ. CT and sonographically guided needle biopsy: current techniques and new innovations. AJR Am J Roentgenol. 1990; 154:1–10.6. Lipnik AJ, Brown DB. Image-guided percutaneous abdominal mass biopsy: technical and clinical considerations. Radiol Clin North Am. 2015; 53:1049–1059.7. Uppot RN, Harisinghani MG, Gervais DA. Imaging-guided percutaneous renal biopsy: rationale and approach. AJR Am J Roentgenol. 2010; 194:1443–1449.8. Atwell TD, Smith RL, Hesley GK, Callstrom MR, Schleck CD, Harmsen WS, et al. Incidence of bleeding after 15,181 percutaneous biopsies and the role of aspirin. AJR Am J Roentgenol. 2010; 194:784–789.9. O'Connor SD, Taylor AJ, Williams EC, Winter TC. Coagulation concepts update. AJR Am J Roentgenol. 2009; 193:1656–1664.10. Burger W, Chemnitius JM, Kneissl GD, Rücker G. Low-dose aspirin for secondary cardiovascular prevention-cardiovascular risks after its perioperative withdrawal versus bleeding risks with its continuation-review and meta-analysis. J Intern Med. 2005; 257:399–414.11. Akan H, Ozen N, Incesu L, Gümüş S, Güneş M. Are percutaneous transgastric biopsies using 14-, 16- and 18-G Tru-Cut needles safe? An experimental study in the rabbit. Australas Radiol. 1998; 42:99–101.12. Brandt KR, Charboneau JW, Stephens DH, Welch TJ, Goellner JR. CT- and US-guided biopsy of the pancreas. Radiology. 1993; 187:99–104.13. Eckman MH, Erban JK, Singh SK, Kao GS. Screening for the risk for bleeding or thrombosis. Ann Intern Med. 2003; 138:W15–W24.14. Sammon J, Twomey M, Crush L, Maher MM, O'Connor OJ. Image-guided percutaneous splenic biopsy and drainage. Semin Intervent Radiol. 2012; 29:301–310.15. Stewart CJ, Coldewey J, Stewart IS. Comparison of fine needle aspiration cytology and needle core biopsy in the diagnosis of radiologically detected abdominal lesions. J Clin Pathol. 2002; 55:93–97.16. Li L, Liu LZ, Wu QL, Mo YX, Liu XW, Cui CY, et al. CT-guided core needle biopsy in the diagnosis of pancreatic diseases with an automated biopsy gun. J Vasc Interv Radiol. 2008; 19:89–94.17. Zamboni GA, D'Onofrio M, Idili A, Malagò R, Iozzia R, Manfrin E, et al. Ultrasound-guided percutaneous fine-needle aspiration of 545 focal pancreatic lesions. AJR Am J Roentgenol. 2009; 193:1691–1695.18. Paulsen SD, Nghiem HV, Negussie E, Higgins EJ, Caoili EM, Francis IR. Evaluation of imaging-guided core biopsy of pancreatic masses. AJR Am J Roentgenol. 2006; 187:769–772.19. Olson MC, Atwell TD, Harmsen WS, Konrad A, King RL, Lin Y, et al. Safety and accuracy of percutaneous image-guided core biopsy of the spleen. AJR Am J Roentgenol. 2016; 206:655–659.20. Appelbaum L, Kane RA, Kruskal JB, Romero J, Sosna J. Focal hepatic lesions: US-guided biopsy--lessons from review of cytologic and pathologic examination results. Radiology. 2009; 250:453–458.21. Perez-Johnston R, Hahn PF, Shenoy-Bhangle AS, Shelly MJ, Gervais DA, Arellano RS. Percutaneous biopsy of focal lesions of the gastrointestinal tract. Abdom Imaging. 2013; 38:1197–1202.22. Hatfield MK, Beres RA, Sane SS, Zaleski GX. Percutaneous imaging-guided solid organ core needle biopsy: coaxial versus noncoaxial method. AJR Am J Roentgenol. 2008; 190:413–417.23. Yoshimatsu R, Yamagami T, Tanaka O, Miura H, Tanaka T, Suzuki T, et al. Comparison of fully automated and semi-automated biopsy needles for lung biopsy under CT fluoroscopic guidance. Br J Radiol. 2012; 85:208–213.24. Sridharan R, Yunos SM, Aziz S, Hussain RI, Alhabshi SM, Suria Hayati MP, et al. Comparison on the use of semi-automated and automated core biopsy needle in ultrasound guided breast biopsy. Med J Malaysia. 2015; 70:326–333.25. Hopper KD, Abendroth CS, Sturtz KW, Matthews YL, Shirk SJ, Stevens LA. Blinded comparison of biopsy needles and automated devices in vitro: 1. Biopsy of diffuse hepatic disease. AJR Am J Roentgenol. 1993; 161:1293–1297.26. Khati NJ, Gorodenker J, Hill MC. Ultrasound-guided biopsies of the abdomen. Ultrasound Q. 2011; 27:255–268.27. Meier-Meitinger M, Anders K, Alibek S, Uder M, Baum U. CT-guided biopsies of pancreatic lesions: impact of contrast application prior to versus following needle placement. Acad Radiol. 2009; 16:1386–1392.28. Longo JM, Bilbao JI, Barettino MD, Larrea JA, Pueyo J, Idoate F, et al. Percutaneous vascular and nonvascular puncture under US guidance: role of color Doppler imaging. Radiographics. 1994; 14:959–972.29. Tang S, Li JH, Lui SL, Chan TM, Cheng IK, Lai KN. Free-hand, ultrasound-guided percutaneous renal biopsy: experience from a single operator. Eur J Radiol. 2002; 41:65–69.30. Phal PM, Brooks DM, Wolfe R. Sonographically guided biopsy of focal lesions: a comparison of freehand and probe-guided techniques using a phantom. AJR Am J Roentgenol. 2005; 184:1652–1656.31. Yu SC, Lau WY, Leung WT, Liew CT, Leung NW, Metreweli C. Percutaneous biopsy of small hepatic lesions using an 18 gauge automated needle. Br J Radiol. 1998; 71:621–624.32. Bisceglia M, Matalon TA, Silver B. The pump maneuver: an atraumatic adjunct to enhance US needle tip localization. Radiology. 1990; 176:867–868.33. Feld R, Needleman L, Goldberg BB. Use of needle-vibrating device and color Doppler imaging for sonographically guided invasive procedures. AJR Am J Roentgenol. 1997; 168:255–256.34. Tseng HS, Chen CY, Chan WP, Chiang JH. Percutaneous transgastric computed tomography-guided biopsy of the pancreas using large needles. World J Gastroenterol. 2009; 15:5972–5975.35. Kim KW, Kim MJ, Kim HC, Park SH, Kim SY, Park MS, et al. Value of “patent track” sign on Doppler sonography after percutaneous liver biopsy in detection of postbiopsy bleeding: a prospective study in 352 patients. AJR Am J Roentgenol. 2007; 189:109–116.36. Weigand K, Weigand K. Percutaneous liver biopsy: retrospective study over 15 years comparing 287 inpatients with 428 outpatients. J Gastroenterol Hepatol. 2009; 24:792–799.37. Micames C, Jowell PS, White R, Paulson E, Nelson R, Morse M, et al. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc. 2003; 58:690–695.38. Horwhat JD, Paulson EK, McGrath K, Branch MS, Baillie J, Tyler D, et al. A randomized comparison of EUS-guided FNA versus CT or US-guided FNA for the evaluation of pancreatic mass lesions. Gastrointest Endosc. 2006; 63:966–975.39. Erturk SM, Mortelé KJ, Tuncali K, Saltzman JR, Lao R, Silverman SG. Fine-needle aspiration biopsy of solid pancreatic masses: comparison of CT and endoscopic sonography guidance. AJR Am J Roentgenol. 2006; 187:1531–1535.40. Zech CJ, Helmberger T, Wichmann MW, Holzknecht N, Diebold J, Reiser MF. Large core biopsy of the pancreas under CT fluoroscopy control: results and complications. J Comput Assist Tomogr. 2002; 26:743–749.41. Gupta S, Ahrar K, Morello FA Jr, Wallace MJ, Hicks ME. Masses in or around the pancreatic head: CT-guided coaxial fine-needle aspiration biopsy with a posterior transcaval approach. Radiology. 2002; 222:63–69.42. Sofocleous CT, Schubert J, Brown KT, Brody LA, Covey AM, Getrajdman GI. CT-guided transvenous or transcaval needle biopsy of pancreatic and peripancreatic lesions. J Vasc Interv Radiol. 2004; 15:1099–1104.43. McInnes MD, Kielar AZ, Macdonald DB. Percutaneous image-guided biopsy of the spleen: systematic review and meta-analysis of the complication rate and diagnostic accuracy. Radiology. 2011; 260:699–708.44. Keogan MT, Freed KS, Paulson EK, Nelson RC, Dodd LG. Imaging-guided percutaneous biopsy of focal splenic lesions: update on safety and effectiveness. AJR Am J Roentgenol. 1999; 172:933–937.45. Venkataramu NK, Sood BP, Gupta S, Gulati M, Khandelwal N, Suri S. Ultrasound-guided fine needle aspiration biopsy of gall bladder malignancies. Acta Radiol. 1999; 40:436–439.46. Pandey M, Sood BP, Shukla RC, Aryya NC, Singh S, Shukla VK. Carcinoma of the gallbladder: role of sonography in diagnosis and staging. J Clin Ultrasound. 2000; 28:227–232.47. Whittier WL, Korbet SM. Renal biopsy: update. Curr Opin Nephrol Hypertens. 2004; 13:661–665.48. Lebret T, Poulain JE, Molinie V, Herve JM, Denoux Y, Guth A, et al. Percutaneous core biopsy for renal masses: indications, accuracy and results. J Urol. 2007; 178(4 Pt 1):1184–1188. discussion 1188.49. Vanderveen KA, Thompson SM, Callstrom MR, Young WF Jr, Grant CS, Farley DR, et al. Biopsy of pheochromocytomas and paragangliomas: potential for disaster. Surgery. 2009; 146:1158–1166.50. Casola G, Nicolet V, vanSonnenberg E, Withers C, Bretagnolle M, Saba RM, et al. Unsuspected pheochromocytoma: risk of blood-pressure alterations during percutaneous adrenal biopsy. Radiology. 1986; 159:733–735.51. Carson BW, Brown JA, Cooperberg PL. Ultrasonographically guided percutaneous biopsy of gastric, small bowel, and colonic abnormalities: efficacy and safety. J Ultrasound Med. 1998; 17:739–742.52. Farmer KD, Harries SR, Fox BM, Maskell GF, Farrow R. Core biopsy of the bowel wall: efficacy and safety in the clinical setting. AJR Am J Roentgenol. 2000; 175:1627–1630.53. Marco-Doménech SF, Gil-Sánchez S, Fernández-García P, De La Iglesia-Carreña P, Gonzalez-Añón M, Arenas-Jimenez JJ, et al. Sonographically guided percutaneous biopsy of gastrointestinal tract lesions. AJR Am J Roentgenol. 2001; 176:147–151.54. Puylaert JB. Acute appendicitis: US evaluation using graded compression. Radiology. 1986; 158:355–360.55. Memel DS, Dodd GD 3rd, Esola CC. Efficacy of sonography as a guidance technique for biopsy of abdominal, pelvic, and retroperitoneal lymph nodes. AJR Am J Roentgenol. 1996; 167:957–962.56. Fisher AJ, Paulson EK, Sheafor DH, Simmons CM, Nelson RC. Small lymph nodes of the abdomen, pelvis, and retroperitoneum: usefulness of sonographically guided biopsy. Radiology. 1997; 205:185–190.57. Spencer JA, Weston MJ, Saidi SA, Wilkinson N, Hall GD. Clinical utility of image-guided peritoneal and omental biopsy. Nat Rev Clin Oncol. 2010; 7:623–631.58. Que Y, Wang X, Liu Y, Li P, Ou G, Zhao W. Ultrasound-guided biopsy of greater omentum: an effective method to trace the origin of unclear ascites. Eur J Radiol. 2009; 70:331–335.59. Lee JK, Baek SY, Lim SM, Lee KH. Reticular infiltrations alone without mass in the mesentery and omentum identified at contrast-enhanced CT: efficacy of US-guided percutaneous core biopsy. Radiology. 2011; 261:311–317.60. Smith EH. Complications of percutaneous abdominal fine-needle biopsy. Review. Radiology. 1991; 178:253–258.61. Tan KT, Rajan DK, Kachura JR, Hayeems E, Simons ME, Ho CS. Pain after percutaneous liver biopsy for diffuse hepatic disease: a randomized trial comparing subcostal and intercostal approaches. J Vasc Interv Radiol. 2005; 16:1215–1219.62. Mueller PR, Biswal S, Halpern EF, Kaufman JA, Lee MJ. Interventional radiologic procedures: patient anxiety, perception of pain, understanding of procedure, and satisfaction with medication--a prospective study. Radiology. 2000; 215:684–688.63. Ralls PW, Barakos JA, Kaptein EM, Friedman PE, Fouladian G, Boswell WD, et al. Renal biopsy-related hemorrhage: frequency and comparison of CT and sonography. J Comput Assist Tomogr. 1987; 11:1031–1034.64. Haaga JR, LiPuma JP, Bryan PJ, Balsara VJ, Cohen AM. Clinical comparison of small-and large-caliber cutting needles for biopsy. Radiology. 1983; 146:665–667.65. Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. American Association for the Study of Liver Diseases. Liver biopsy. Hepatology. 2009; 49:1017–1044.66. Martino CR, Haaga JR, Bryan PJ, LiPuma JP, El Yousef SJ, Alfidi RJ. CT-guided liver biopsies: eight years' experience. Work in progress. Radiology. 1984; 152:755–757.67. Elvin A, Andersson T, Scheibenpflug L, Lindgren PG. Biopsy of the pancreas with a biopsy gun. Radiology. 1990; 176:677–679.68. Chang S, Kim SH, Lim HK, Lee WJ, Choi D, Lim JH. Needle tract implantation after sonographically guided percutaneous biopsy of hepatocellular carcinoma: evaluation of doubling time, frequency, and features on CT. AJR Am J Roentgenol. 2005; 185:400–405.69. Ferrucci JT, Wittenberg J, Margolies MN, Carey RW. Malignant seeding of the tract after thin-needle aspiration biopsy. Radiology. 1979; 130:345–346.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Usefulness of Ultrasound-Guided Automated Core Biopsy of Nonpalpable Breast Lesions
- Pseudoaneurysm of the Breast after Core Needle Biopsy: A Case Report
- Fine-Needle Biopsy: Should This Be the First Choice in Endoscopic Ultrasound-Guided Tissue Acquisition?
- Breast Lesions with Discordant Results on Ultrasound-guided Core Needle Biopsy
- Endoscopic Ultrasound-Fine Needle Aspiration versus Core Biopsy for the Diagnosis of Subepithelial Tumors