J Pathol Transl Med.
2018 Nov;52(6):404-410. 10.4132/jptm.2018.09.20.
The Usefulness of Immunocytochemistry of CD56 in Determining Malignancy from Indeterminate Thyroid Fine-Needle Aspiration Cytology
- Affiliations
-
- 1Department of Pathology, Gangnam Severance Hospital, Seoul, Korea. SOONWONH@yuhs.ac
- KMID: 2427523
- DOI: http://doi.org/10.4132/jptm.2018.09.20
Abstract
- BACKGROUND
Fine-needle aspiration cytology serves as a safe, economical tool in evaluating thyroid nodules. However, about 30% of the samples are categorized as indeterminate. Hence, many immunocytochemistry markers have been studied, but there has not been a single outstanding marker. We studied the efficacy of CD56 with human bone marrow endothelial cell marker-1 (HBME-1) in diagnosis in the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) category III.
METHODS
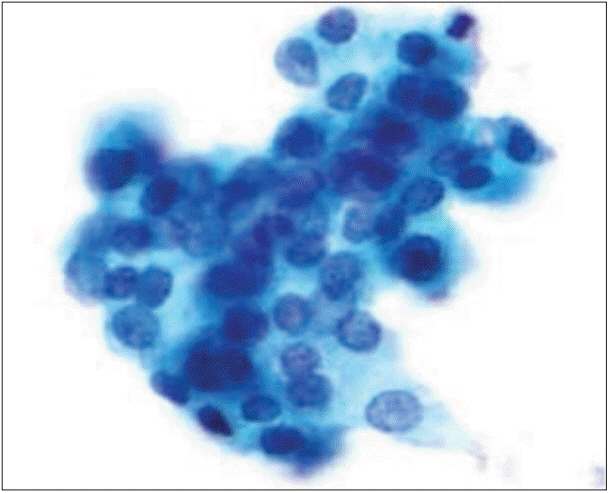
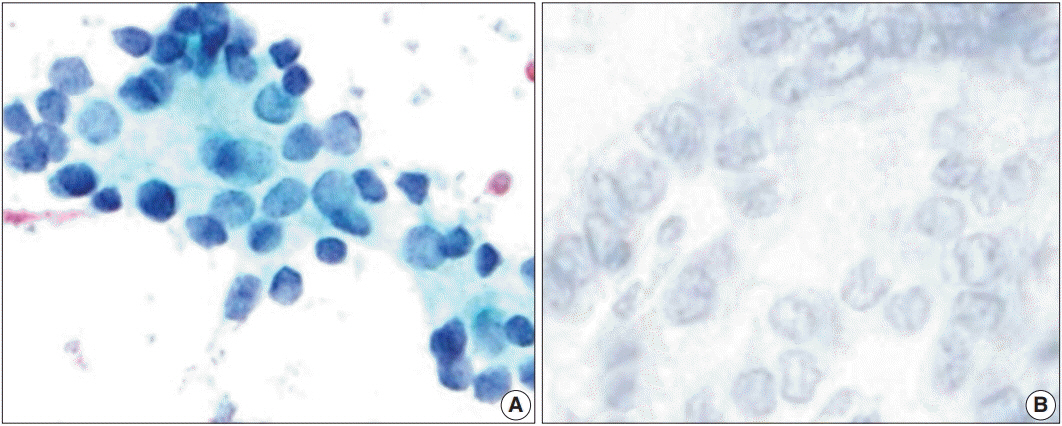
We reviewed ThinPrep liquid-based cytology (LBC) samples with Papanicolaou stain from July 1 to December 31, 2016 (2,195 cases) and selected TBSRTC category III cases (n = 363). Twenty-six cases were histologically confirmed as benign (six cases, 23%) or malignant (20 cases, 77%); we stained 26 LBC slides with HBME-1 and CD56 through the cell transfer method. For evaluation of reactivity of immunocytochemistry, we chose atypical follicular cell clusters.
RESULTS
CD56 was not reactive in 18 of 20 cases (90%) of malignant nodules and showed cytoplasmic positivity in five of six cases (83%) of benign nodules. CD56 showed high sensitivity (90.0%) and relatively low specificity (83.3%) in detecting malignancy (p = .004). HBME-1 was reactive in 17 of 20 cases (85%) of malignant nodules and was not reactive in five of six cases (83%) of benign nodules. HBME-1 showed slightly lower sensitivity (85.0%) than CD56. The specificity in detecting malignancy by HBME-1 was similar to that of CD56 (83.3%, p = .008). CD56 and HBME-1 tests combined showed lower sensitivity (75.0% vs 90%) and higher specificity (93.8% vs 83.3%) in detecting malignancy compared to using CD56 alone.
CONCLUSIONS
Using CD56 alone showed relatively low specificity despite high sensitivity for detecting malignancy. Combining CD56 with HBME-1 could increase the specificity. Thus, we suggest that CD56 could be a useful preoperative marker for differential diagnosis of TBSRTC category III samples.
Keyword
MeSH Terms
Figure
Reference
-
1. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19:1167–214.
Article2. Ahn HS, Kim HJ, Kim KH, et al. Thyroid cancer screening in South Korea increases detection of papillary cancers with no impact on other subtypes or thyroid cancer mortality. Thyroid. 2016; 26:1535–40.
Article3. Cha YJ, Pyo JY, Hong S, et al. Thyroid fine-needle aspiration cytology practice in Korea. J Pathol Transl Med. 2017; 51:521–7.
Article4. Powsner SM, Costa J, Homer RJ. Clinicians are from Mars and pathologists are from Venus. Arch Pathol Lab Med. 2000; 124:1040–6.
Article5. Cibas ES, Ali SZ; NCI Thyroid FNA State of the Science Conference. The Bethesda System For Reporting Thyroid Cytopathology. Am J Clin Pathol. 2009; 132:658–65.
Article6. Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2017; 27:1341–6.
Article7. Jung CK, Hong S, Bychkov A, Kakudo K. The use of fine-needle aspiration (FNA) cytology in patients with thyroid nodules in Asia: a brief overview of studies from the Working Group of Asian Thyroid FNA Cytology. J Pathol Transl Med. 2017; 51:571–8.
Article8. Kang Y, Lee YJ, Jung J, Lee Y, Won NH, Chae YS. Morphometric analysis of thyroid follicular cells with atypia of undetermined significance. J Pathol Transl Med. 2016; 50:287–93.
Article9. Yoo C, Choi HJ, Im S, et al. Fine needle aspiration cytology of thyroid follicular neoplasm: cytohistologic correlation and accuracy. Korean J Pathol. 2013; 47:61–6.
Article10. Oosthuizen JL, Walker B, Todorovic E, Masoudi H, Wiseman SM. The presence of papillary features in thyroid nodules diagnosed as atypia of undetermined significance or follicular lesion of undetermined significance increases cancer risk and should influence treatment. Am J Surg. 2018; 215:819–23.
Article11. Yashaswini R, Suresh TN, Sagayaraj A. Cytological evaluation of thyroid lesions by nuclear morphology and nuclear morphometry. J Cytol. 2017; 34:197–202.
Article12. Vivero M, Renshaw AA, Krane JF. Adequacy criteria for thyroid FNA evaluated by ThinPrep slides only. Cancer Cytopathol. 2017; 125:534–43.
Article13. Rossi M, Lupo S, Rossi R, et al. Proposal for a novel management of indeterminate thyroid nodules on the basis of cytopathological subclasses. Endocrine. 2017; 57:98–107.
Article14. Satoh S, Yamashita H, Kakudo K. Thyroid cytology: The Japanese system and experience at Yamashita Thyroid Hospital. J Pathol Transl Med. 2017; 51:548–54.
Article15. Kim SJ, Roh J, Baek JH, et al. Risk of malignancy according to subclassification of the atypia of undetermined significance or follicular lesion of undetermined significance (AUS/FLUS) category in the Bethesda system for reporting thyroid cytopathology. Cytopathology. 2017; 28:65–73.
Article16. Misiakos EP, Margari N, Meristoudis C, et al. Cytopathologic diagnosis of fine needle aspiration biopsies of thyroid nodules. World J Clin Cases. 2016; 4:38–48.
Article17. Kholová I, Ludvíková M. Thyroid atypia of undetermined significance or follicular lesion of undetermined significance: an indispensable Bethesda 2010 diagnostic category or waste garbage? Acta Cytol. 2014; 58:319–29.
Article18. Shi Y, Ding X, Klein M, et al. Thyroid fine-needle aspiration with atypia of undetermined significance: a necessary or optional category? Cancer. 2009; 117:298–304.19. Garg S, Naik LP, Kothari KS, Fernandes GC, Agnihotri MA, Gokhale JC. Evaluation of thyroid nodules classified as Bethesda category III on FNAC. J Cytol. 2017; 34:5–9.
Article20. Rossi ED, Martini M, Capodimonti S, et al. Diagnostic and prognostic value of immunocytochemistry and BRAF mutation analysis on liquid-based biopsies of thyroid neoplasms suspicious for carcinoma. Eur J Endocrinol. 2013; 168:853–9.
Article21. Fadda G, Rossi ED, Raffaelli M, et al. Follicular thyroid neoplasms can be classified as low- and high-risk according to HBME-1 and Galectin-3 expression on liquid-based fine-needle cytology. Eur J Endocrinol. 2011; 165:447–53.
Article22. Ohori NP, Nikiforova MN, Schoedel KE, et al. Contribution of molecular testing to thyroid fine-needle aspiration cytology of “follicular lesion of undetermined significance/atypia of undetermined significance”. Cancer Cytopathol. 2010; 118:17–23.
Article23. Cochand-Priollet B, Dahan H, Laloi-Michelin M, et al. Immunocytochemistry with cytokeratin 19 and anti-human mesothelial cell antibody (HBME1) increases the diagnostic accuracy of thyroid fineneedle aspirations: preliminary report of 150 liquid-based fine-needle aspirations with histological control. Thyroid. 2011; 21:1067–73.
Article24. Abouhashem NS, Talaat SM. Diagnostic utility of CK19 and CD56 in the differentiation of thyroid papillary carcinoma from its mimics. Pathol Res Pract. 2017; 213:509–17.
Article25. Solmaz OA. Diagnostic importance of CD56 with fine-needle aspiration cytology in suspected papillary thyroid carcinoma cases. Cytojournal. 2018; 15:3.
Article26. Mokhtari M, Eftekhari M, Tahririan R. Absent CD56 expression in papillary thyroid carcinoma: A finding of potential diagnostic value in problematic cases of thyroid pathology. J Res Med Sci. 2013; 18:1046–50.27. Nechifor-Boila A, Borda A, Sassolas G, et al. Immunohistochemical markers in the diagnosis of papillary thyroid carcinomas: the promising role of combined immunostaining using HBME-1 and CD56. Pathol Res Pract. 2013; 209:585–92.
Article28. Nechifor-Boila˘ A, Ca˘tana˘ R, Loghin A, Radu TG, Borda A. Diagnostic value of HBME-1, CD56, galectin-3 and cytokeratin-19 in papillary thyroid carcinomas and thyroid tumors of uncertain malignant potential. Rom J Morphol Embryol. 2014; 55:49–56.29. Scarpino S, Di Napoli A, Melotti F, Talerico C, Cancrini A, Ruco L. Papillary carcinoma of the thyroid: low expression of NCAM (CD56) is associated with downregulation of VEGF-D production by tumour cells. J Pathol. 2007; 212:411–9.
Article30. Shahebrahimi K, Madani SH, Fazaeli AR, Khazaei S, Kanani M, Keshavarz A. Diagnostic value of CD56 and nm23 markers in papillary thyroid carcinoma. Indian J Pathol Microbiol. 2013; 56:2–5.
Article31. Huang L, Wang X, Huang X, et al. Diagnostic significance of CK19, galectin-3, CD56, TPO and Ki67 expression and BRAF mutation in papillary thyroid carcinoma. Oncol Lett. 2018; 15:4269–77.
Article32. Muzafar A, Bukhari MH, Qureshi IU. A study of galactin-3 on fine needle aspiration as a diagnostic marker differentiating benign from malignant thyroid neoplasm. Pak J Med Sci. 2017; 33:726–31.
Article33. Golu I, Vlad MM, Dema A, et al. The absence of CD56 expression can differentiate papillary thyroid carcinoma from other thyroid lesions. Indian J Pathol Microbiol. 2017; 60:161–6.
Article34. Abd El Atti RM, Shash LS. Potential diagnostic utility of CD56 and claudin-1 in papillary thyroid carcinoma and solitary follicular thyroid nodules. J Egypt Natl Canc Inst. 2012; 24:175–84.
Article35. Ceyran AB, S¸enol S, S¸ims¸ek BÇ, Sag˘ırog˘lu J, Aydın A. Role of cd56 and e-cadherin expression in the differential diagnosis of papillary thyroid carcinoma and suspected follicular-patterned lesions of the thyroid: the prognostic importance of e-cadherin. Int J Clin Exp Pathol. 2015; 8:3670–80.36. Erdogan-Durmus S, Ozcan D, Yarikkaya E, Kurt A, Arslan A. CD56, HBME-1 and cytokeratin 19 expressions in papillary thyroid carcinoma and nodular thyroid lesions. J Res Med Sci. 2016; 21:49.
Article37. Oh EJ, Hong SW, Jeong HJ, Yoon SO. The diagnostic approach to fine-needle aspiration of malignant lymphoma: using cytomorphology and immunocytochemistry with cell transfer method. Diagn Cytopathol. 2014; 42:671–9.
Article38. Sherman ME, Jimenez-Joseph D, Gangi MD, Rojas-Corona RR. Immunostaining of small cytologic specimens: facilitation with cell transfer. Acta Cytol. 1994; 38:18–22.39. Zu Y, Gangi MD, Yang GC. Ultrafast Papanicolaou stain and cell-transfer technique enhance cytologic diagnosis of Hodgkin lymphoma. Diagn Cytopathol. 2002; 27:308–11.
Article40. El Demellawy D, Nasr AL, Babay S, Alowami S. Diagnostic utility of CD56 immunohistochemistry in papillary carcinoma of the thyroid. Pathol Res Pract. 2009; 205:303–9.
Article41. Zeromski J, Bagnasco M, Paolieri F, Dworacki G. Expression of CD56 (NKH-1) differentiation antigen in human thyroid epithelium. Clin Exp Immunol. 1992; 89:474–8.
Article42. Ozolins A, Narbuts Z, Strumfa I, Volanska G, Stepanovs K, Gardovskis J. Immunohistochemical expression of HBME-1, E-cadherin, and CD56 in the differential diagnosis of thyroid nodules. Medicina (Kaunas). 2012; 48:507–14.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- US Diagnosis for Thyroid Nodules with an Indeterminate Cytology
- The Analysis of Indeterminate Category in Thyroid Fine Needle Aspiration
- Thyroid Fine-Needle Aspiration in Taiwan: The History and Current Practice
- Indications for Fine Needle Aspiration in Thyroid Nodules
- Thyroid nodules with discordant results of ultrasonographic and fine-needle aspiration findings