Novel Application of Quantitative Single-Photon Emission Computed Tomography/Computed Tomography to Predict Early Response to Methimazole in Graves' Disease
- Affiliations
-
- 1Department of Nuclear Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam 13620, Korea. wwlee@snu.ac.kr
- 2Department of Molecular Medicine and Biopharmaceutical Sciences, Graduate School of Convergence Science and Technology, Seoul National University, Suwon 16229, Korea.
- 3Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam 13620, Korea.
- 4Department of Nuclear Medicine, Konkuk University Medical Center, Seoul 05030, Korea.
- 5Institute of Radiation Medicine, Medical Research Center, Seoul National University, Seoul 08826, Korea.
- KMID: 2427310
- DOI: http://doi.org/10.3348/kjr.2017.18.3.543
Abstract
OBJECTIVE
Since Graves' disease (GD) is resistant to antithyroid drugs (ATDs), an accurate quantitative thyroid function measurement is required for the prediction of early responses to ATD. Quantitative parameters derived from the novel technology, single-photon emission computed tomography/computed tomography (SPECT/CT), were investigated for the prediction of achievement of euthyroidism after methimazole (MMI) treatment in GD.
MATERIALS AND METHODS
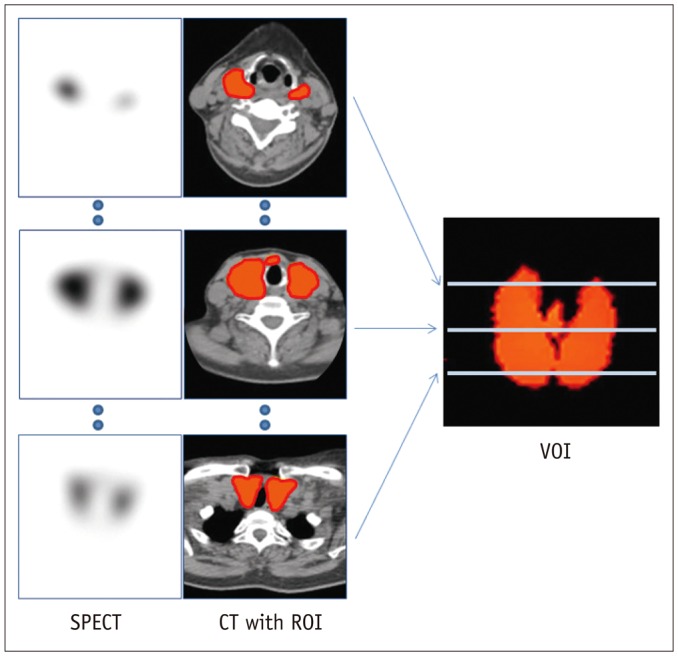
A total of 36 GD patients (10 males, 26 females; mean age, 45.3 ± 13.8 years) were enrolled for this study, from April 2015 to January 2016. They underwent quantitative thyroid SPECT/CT 20 minutes post-injection of (99m)Tc-pertechnetate (5 mCi). Association between the time to biochemical euthyroidism after MMI treatment and %uptake, standardized uptake value (SUV), functional thyroid mass (SUVmean × thyroid volume) from the SPECT/CT, and clinical/biochemical variables, were investigated.
RESULTS
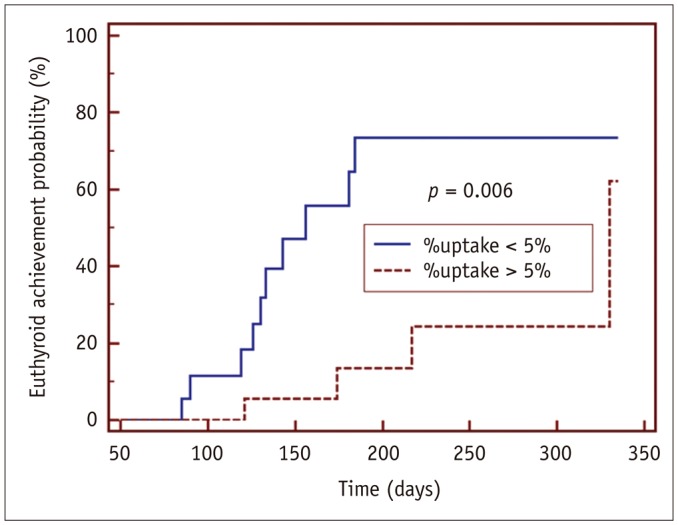
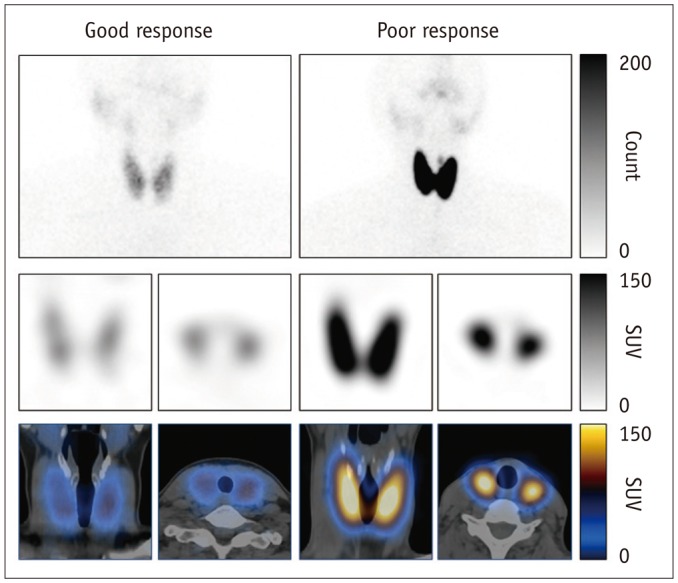
GD patients had a significantly greater %uptake (6.9 ± 6.4%) than historical control euthyroid patients (n = 20, 0.8 ± 0.5%, p < 0.001) from the same quantitative SPECT/CT protocol. Euthyroidism was achieved in 14 patients at 156 ± 62 days post-MMI treatment, but 22 patients had still not achieved euthyroidism by the last follow-up time-point (208 ± 80 days). In the univariate Cox regression analysis, the initial MMI dose (p = 0.014), %uptake (p = 0.015), and functional thyroid mass (p = 0.016) were significant predictors of euthyroidism in response to MMI treatment. However, only %uptake remained significant in a multivariate Cox regression analysis (p = 0.034). A %uptake cutoff of 5.0% dichotomized the faster responding versus the slower responding GD patients (p = 0.006).
CONCLUSION
A novel parameter of thyroid %uptake from quantitative SPECT/CT is a predictive indicator of an early response to MMI in GD patients.
Keyword
MeSH Terms
-
Adult
Antithyroid Agents/therapeutic use
Female
Graves Disease/*diagnostic imaging/drug therapy/pathology
Humans
Male
Methimazole/therapeutic use
Middle Aged
Proportional Hazards Models
Sodium Pertechnetate Tc 99m/chemistry
Thyrotropin/analysis
*Tomography, Emission-Computed, Single-Photon
Antithyroid Agents
Methimazole
Thyrotropin
Sodium Pertechnetate Tc 99m
Figure
Cited by 4 articles
-
RE: Novel Application of Quantitative Single-Photon Emission Computed Tomography/Computed Tomography to Predict Early Response to Methimazole in Graves' Disease
Wei Zhang, Zhuo-Qun Huang, Wei-Long Lin, Shi-Hong Yang
Korean J Radiol. 2018;19(1):185-186. doi: 10.3348/kjr.2018.19.1.185.RE: Novel Application of Quantitative Single-Photon Emission Computed Tomography/Computed Tomography to Predict Early Response to Methimazole in Graves' Disease
Wei Zhang, Zhuo-Qun Huang, Wei-Long Lin, Shi-Hong Yang
Korean J Radiol. 2018;19(1):185-186. doi: 10.3348/kjr.2018.19.1.185.Age of Data in Contemporary Research Articles Published in Representative General Radiology Journals
Ji Hun Kang, Dong Hwan Kim, Seong Ho Park, Jung Hwan Baek
Korean J Radiol. 2018;19(6):1172-1178. doi: 10.3348/kjr.2018.19.6.1172.Utility of Quantitative Parameters from Single-Photon Emission Computed Tomography/Computed Tomography in Patients with Destructive Thyroiditis
Ji-Young Kim, Ji Hyun Kim, Jae Hoon Moon, Kyoung Min Kim, Tae Jung Oh, Dong-Hwa Lee, Young So, Won Woo Lee
Korean J Radiol. 2018;19(3):470-480. doi: 10.3348/kjr.2018.19.3.470.
Reference
-
1. Brent GA. Clinical practice. Graves' disease. N Engl J Med. 2008; 358:2594–2605. PMID: 18550875.2. Bahn Chair RS, Burch HB, Cooper DS, Garber JR, Greenlee MC, Klein I, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid. 2011; 21:593–646. PMID: 21510801.
Article3. Törring O, Tallstedt L, Wallin G, Lundell G, Ljunggren JG, Taube A, et al. Graves' hyperthyroidism: treatment with antithyroid drugs, surgery, or radioiodine--a prospective, randomized study. Thyroid Study Group. J Clin Endocrinol Metab. 1996; 81:2986–2993. PMID: 8768863.
Article4. Burch HB, Cooper DS. Management of Graves disease: a review. JAMA. 2015; 314:2544–2554. PMID: 26670972.5. Vitti P, Rago T, Chiovato L, Pallini S, Santini F, Fiore E, et al. Clinical features of patients with Graves' disease undergoing remission after antithyroid drug treatment. Thyroid. 1997; 7:369–375. PMID: 9226205.
Article6. Allahabadia A, Daykin J, Holder RL, Sheppard MC, Gough SC, Franklyn JA. Age and gender predict the outcome of treatment for Graves' hyperthyroidism. J Clin Endocrinol Metab. 2000; 85:1038–1042. PMID: 10720036.
Article7. Abraham P, Avenell A, Park CM, Watson WA, Bevan JS. A systematic review of drug therapy for Graves' hyperthyroidism. Eur J Endocrinol. 2005; 153:489–498. PMID: 16189168.
Article8. Benker G, Vitti P, Kahaly G, Raue F, Tegler L, Hirche H, et al. The European Multicenter Study Group. Response to methimazole in Graves' disease. Clin Endocrinol (Oxf). 1995; 43:257–263. PMID: 7586593.
Article9. Madec AM, Laurent MC, Lorcy Y, Le Guerrier AM, Rostagnat-Stefanutti A, Orgiazzi J, et al. Thyroid stimulating antibodies: an aid to the strategy of treatment of Graves' disease? Clin Endocrinol (Oxf). 1984; 21:247–255. PMID: 6148163.
Article10. Glinoer D, de Nayer P, Bex M. Belgian Collaborative Study Group on Graves' Disease. Effects of l-thyroxine administration, TSH-receptor antibodies and smoking on the risk of recurrence in Graves' hyperthyroidism treated with antithyroid drugs: a double-blind prospective randomized study. Eur J Endocrinol. 2001; 144:475–483. PMID: 11331213.
Article11. Lee H, Kim JH, Kang YK, Moon JH, So Y, Lee WW. Quantitative single-photon emission computed tomography/computed tomography for technetium pertechnetate thyroid uptake measurement. Medicine (Baltimore). 2016; 95:e4170. PMID: 27399139.
Article13. Mazza E, Carlini M, Flecchia D, Blatto A, Zuccarini O, Gamba S, et al. Long-term follow-up of patients with hyperthyroidism due to Graves' disease treated with methimazole. Comparison of usual treatment schedule with drug discontinuation vs continuous treatment with low methimazole doses: a retrospective study. J Endocrinol Invest. 2008; 31:866–872. PMID: 19092290.
Article14. Meller J, Becker W. The continuing importance of thyroid scintigraphy in the era of high-resolution ultrasound. Eur J Nucl Med Mol Imaging. 2002; 29(Suppl 2):S425–S438. PMID: 12192542.
Article15. Chang KJ, Lim I, Park JY, Jo AR, Kong CB, Song WS, et al. The role of (18)F-FDG PET/CT as a prognostic factor in patients with synovial sarcoma. Nucl Med Mol Imaging. 2015; 49:33–41. PMID: 25774236.
Article16. Park S, Lee E, Rhee S, Cho J, Choi S, Lee S, et al. Correlation between semi-quantitative (18)F-FDG PET/CT parameters and Ki-67 expression in small cell lung cancer. Nucl Med Mol Imaging. 2016; 50:24–30. PMID: 26941856.
Article17. Shimmins JG, Harden RM, Alexander WD. Loss of pertechnetate from the human thyroid. J Nucl Med. 1969; 10:637–640. PMID: 5808845.18. Atkins HL, Richards P. Assessment of thyroid function and anatomy with technetium-99m as pertechnetate. J Nucl Med. 1968; 9:7–15. PMID: 5634415.19. Lucas KJ. Use of thyroid ultrasound volume in calculating radioactive iodine dose in hyperthyroidism. Thyroid. 2000; 10:151–155. PMID: 10718551.
Article20. Berghout A, Wiersinga WM, Smits NJ, Touber JL. Determinants of thyroid volume as measured by ultrasonography in healthy adults in a non-iodine deficient area. Clin Endocrinol (Oxf). 1987; 26:273–280. PMID: 3308184.
Article21. Zantut-Wittmann DE, Ramos CD, Santos AO, Lima MM, Panzan AD, Facuri FV, et al. High pre-therapy [99mTc]pertechnetate thyroid uptake, thyroid size and thyrostatic drugs: predictive factors of failure in [131I]iodide therapy in Graves' disease. Nucl Med Commun. 2005; 26:957–963. PMID: 16208172.
Article22. El-Kareem MA, Derwish WA, Moustafa HM. Response rate and factors affecting the outcome of a fixed dose of RAI-131 therapy in Graves' disease: a 10-year Egyptian experience. Nucl Med Commun. 2014; 35:900–907. PMID: 24926901.23. Atkins HL, Fleay RF. Data blending with 99mTc in evaluating thyroid anatomy by scintillation scanning. J Nucl Med. 1968; 9:66–73. PMID: 5635237.24. Lee WW, Lee B, Kim SJ, Jin J, Moon DH, Lee H. Kinetics of iodide uptake and efflux in various human thyroid cancer cells by expressing sodium iodide symporter gene via a recombinant adenovirus. Oncol Rep. 2003; 10:845–849. PMID: 12792733.
Article25. Alexander EK, Larsen PR. High dose of (131)I therapy for the treatment of hyperthyroidism caused by Graves' disease. J Clin Endocrinol Metab. 2002; 87:1073–1077. PMID: 11889166.26. Silberstein EB, Alavi A, Balon HR, Clarke SE, Divgi C, Gelfand MJ, et al. The SNMMI practice guideline for therapy of thyroid disease with 131I 3.0. J Nucl Med. 2012; 53:1633–1651. PMID: 22787108.
Article27. Ritt P, Vija H, Hornegger J, Kuwert T. Absolute quantification in SPECT. Eur J Nucl Med Mol Imaging. 2011; 38(Suppl 1):S69–S77. PMID: 21484383.
Article28. Bailey DL, Willowson KP. An evidence-based review of quantitative SPECT imaging and potential clinical applications. J Nucl Med. 2013; 54:83–89. PMID: 23283563.
Article29. Cachovan M, Vija AH, Hornegger J, Kuwert T. Quantification of 99mTc-DPD concentration in the lumbar spine with SPECT/CT. EJNMMI Res. 2013; 3:45. PMID: 23738809.
Article30. Suh MS, Lee WW, Kim YK, Yun PY, Kim SE. Maximum standardized uptake value of (99m)Tc hydroxymethylene diphosphonate SPECT/CT for the evaluation of temporomandibular joint disorder. Radiology. 2016; 280:890–896. PMID: 27035060.31. Walter MA, Christ-Crain M, Eckard B, Schindler C, Nitzsche EU, Müller-Brand J, et al. Radioiodine therapy in hyperthyroidism: inverse correlation of pretherapeutic iodine uptake level and post-therapeutic outcome. Eur J Clin Invest. 2004; 34:365–370. PMID: 15147334.
Article32. Kristoffersen US, Hesse B, Rasmussen AK, Kjaer A. Radioiodine therapy in hyperthyroid disease: poorer outcome in patients with high 24 hours radioiodine uptake. Clin Physiol Funct Imaging. 2006; 26:167–170. PMID: 16640512.
Article33. Damle N, Bal C, Kumar P, Reddy R, Virkar D. The predictive role of 24h RAIU with respect to the outcome of low fixed dose radioiodine therapy in patients with diffuse toxic goiter. Hormones (Athens). 2012; 11:451–457. PMID: 23422768.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- RE: Novel Application of Quantitative Single-Photon Emission Computed Tomography/Computed Tomography to Predict Early Response to Methimazole in Graves' Disease
- Clinical Applications of Technetium-99m Quantitative Single-Photon Emission Computed Tomography/Computed Tomography
- Nuclear Medicine Imaging in Rheumatic Diseases
- Clinical Application of SPECT and PET in CerebroVascular Disease
- Diagnosis of hepatic hemangioma with 99mTc-labeled red cells and single photon emission computed tomography (SPECT)