Yonsei Med J.
2016 Nov;57(6):1395-1403. 10.3349/ymj.2016.57.6.1395.
Characteristics Predictive for a Successful Switch from Insulin Analogue Therapy to Oral Hypoglycemic Agents in Patients with Type 2 Diabetes
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. bwanlee@yuhs.ac
- 2Graduate School, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2427157
- DOI: http://doi.org/10.3349/ymj.2016.57.6.1395
Abstract
- PURPOSE
The objective of this study was to investigate clinical and laboratory parameters that could predict which patients could maintain adequate glycemic control after switching from initial insulin therapy to oral hypoglycemic agents (OHAs) among patients with type 2 diabetes (T2D).
MATERIALS AND METHODS
We recruited 275 patients with T2D who had been registered in 3 cohorts of initiated insulin therapy and followed up for 33 months. The participants were divided into 2 groups according to whether they switched from insulin to OHAs (Group I) or not (Group II), and Group I was further classified into 2 sub-groups: maintenance on OHAs (Group IA) or resumption of insulin (Group IB).
RESULTS
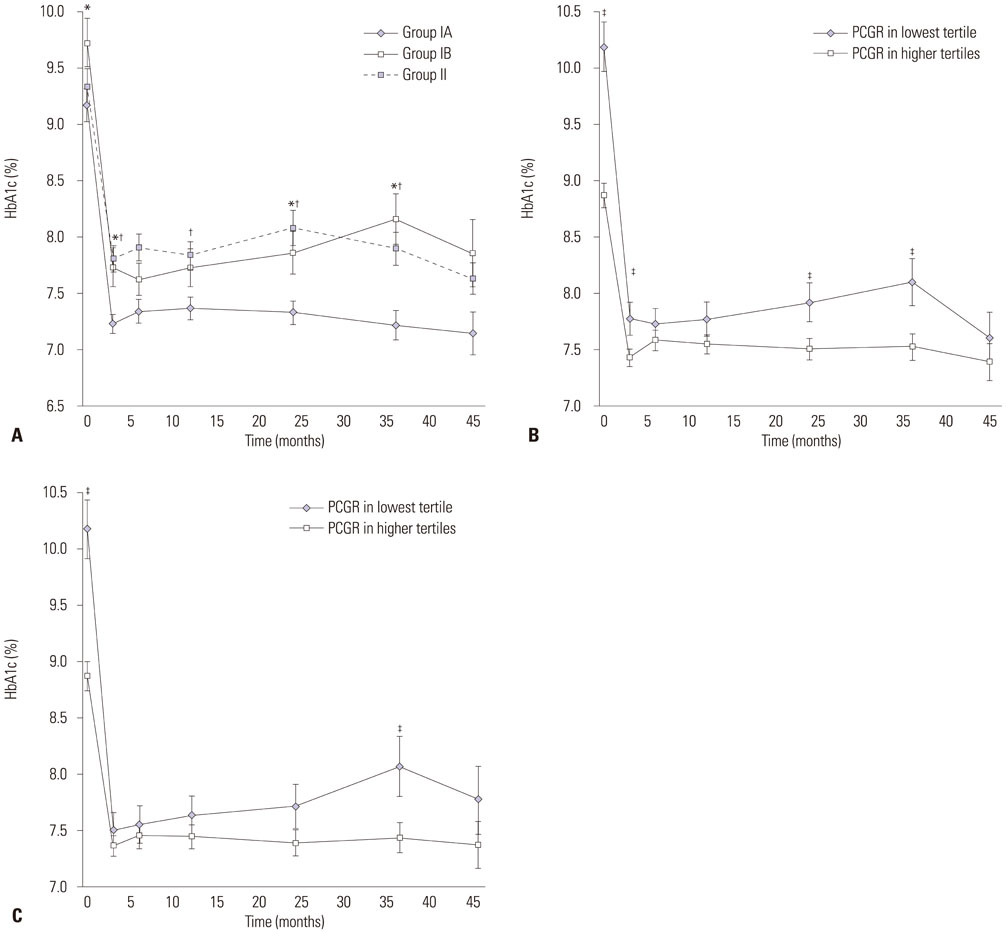
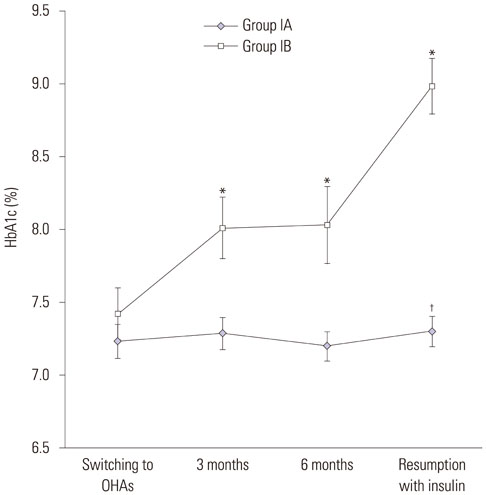
Of 275 patients with insulin initiation, 63% switched to OHAs (Group I) and 37% continued insulin (Group II). Of these, 44% were in Group IA and 19% in Group IB. The lowest tertile of baseline postprandial C-peptide-to-glucose ratio (PCGR), higher insulin dose at switching to OHAs, and higher HbA1c level at 6 months after switching to OHAs were all associated with OHA failure (Group IB; p=0.001, 0.046, and 0.014, respectively). The lowest tertile of PCGR was associated with ultimate use of insulin (Group IB and Group II; p=0.029).
CONCLUSION
Higher baseline level of PCGR and lower HbA1c levels at 6 months after switching to OHAs may be strong predictors for the successful maintenance of OHAs after switching from insulin therapy in Korean patients with T2D.
MeSH Terms
-
Adult
Blood Glucose/*metabolism
C-Peptide
Diabetes Mellitus, Type 2/*drug therapy/metabolism/physiopathology
Drug Therapy, Combination
Female
Glycated Hemoglobin A/analysis/*metabolism
Humans
Hypoglycemic Agents/*administration & dosage/therapeutic use
Insulin/*administration & dosage/therapeutic use
Male
Middle Aged
Postprandial Period
Blood Glucose
C-Peptide
Glycated Hemoglobin A
Hypoglycemic Agents
Insulin
Figure
Reference
-
1. DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988; 37:667–687.2. Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003; 46:3–19.
Article3. Kim YJ, Jeon JY, Han SJ, Kim HJ, Lee KW, Kim DJ. Effect of socio-economic status on the prevalence of diabetes. Yonsei Med J. 2015; 56:641–647.
Article4. Weng J, Li Y, Xu W, Shi L, Zhang Q, Zhu D, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008; 371:1753–1760.
Article5. Kramer CK, Zinman B, Retnakaran R. Short-term intensive insulin therapy in type 2 diabetes mellitus: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2013; 1:28–34.
Article6. Tibaldi J, Rakel RE. Why, when and how to initiate insulin therapy in patients with type 2 diabetes. Int J Clin Pract. 2007; 61:633–644.
Article7. Yoo HJ, Park KY, Park KS, Ahn KJ, Min KW, Park JH, et al. Safety and efficacy of modern insulin analogues. Diabetes Metab J. 2013; 37:181–189.
Article8. Riddle MC, Rosenstock J, Gerich J. Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003; 26:3080–3086.9. Holman RR, Farmer AJ, Davies MJ, Levy JC, Darbyshire JL, Keenan JF, et al. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009; 361:1736–1747.
Article10. Janka HU, Plewe G, Riddle MC, Kliebe-Frisch C, Schweitzer MA, Yki-Järvinen H. Comparison of basal insulin added to oral agents versus twice-daily premixed insulin as initial insulin therapy for type 2 diabetes. Diabetes Care. 2005; 28:254–259.
Article11. Hwang YC, Kang JG, Ahn KJ, Cha BS, Ihm SH, Lee S, et al. The glycemic efficacies of insulin analogue regimens according to baseline glycemic status in Korean patients with type 2 diabetes: sub-analysis from the A(1)chieve(®) study. Int J Clin Pract. 2014; 68:1338–1344.
Article12. Lee YH, Lee BW, Chun SW, Cha BS, Lee HC. Predictive characteristics of patients achieving glycaemic control with insulin after sulfonylurea failure. Int J Clin Pract. 2011; 65:1076–1084.
Article13. Choe EY, Lee YH, Lee BW, Kang ES, Cha BS, Lee HC. Glycemic effects of once-a-day rapid-acting insulin analogue addition on a basal insulin analogue in Korean subjects with poorly controlled type 2 diabetes mellitus. Diabetes Metab J. 2012; 36:230–236.
Article14. Lee YH, Lee BW, Kwon HJ, Kang ES, Cha BS, Lee HC. Higher morning to evening ratio in total dose of twice-daily biphasic insulin analog might be effective in achieving glucose control in patients with poorly controlled type 2 diabetes. Diabetes Technol Ther. 2012; 14:508–514.
Article15. Jung CH, Park JY, Cho JH, Yoon KH, Yang HK, Lee YH, et al. The optimal morning:evening ratio in total dose of twice-daily biphasic insulin analogue in poorly controlled Type 2 diabetes: a 24-week multi-centre prospective, randomized controlled, open-labelled clinical study. Diabet Med. 2014; 31:68–75.
Article16. Kim W, Kim KJ, Lee BW, Kang ES, Cha BS, Lee HC. The glycated albumin to glycated hemoglobin ratio might not be associated with carotid atherosclerosis in patients with type 1 diabetes. Diabetes Metab J. 2014; 38:456–463.
Article17. Yamada Y, Fukuda K, Fujimoto S, Hosokawa M, Tsukiyama K, Nagashima K, et al. SUIT, secretory units of islets in transplantation: An index for therapeutic management of islet transplanted patients and its application to type 2 diabetes. Diabetes Res Clin Pract. 2006; 74:222–226.
Article18. Lee EY, Hwang S, Lee SH, Lee YH, Choi AR, Lee Y, et al. Postprandial C-peptide to glucose ratio as a predictor of β-cell function and its usefulness for staged management of type 2 diabetes. J Diabetes Investig. 2014; 5:517–524.
Article19. Ito R, Fukui T, Hayashi T, Osamura A, Ohara M, Hara N, et al. Teneligliptin, a dipeptidyl peptidase-4 inhibitor, improves early-phase insulin secretion in drug-naïve patients with type 2 diabetes. Drugs R D. 2015; 15:245–251.
Article20. Park SY, Cho YJ, Lee SR, Chung H, Jeong K. Triglyceride is a useful surrogate marker for insulin resistance in Korean women with polycystic ovary syndrome. Yonsei Med J. 2015; 56:785–792.
Article21. World Health Organization, Regional Office for the Western Pacific. The Asia Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia;2000.22. Steil GM, Hwu CM, Janowski R, Hariri F, Jinagouda S, Darwin C, et al. Evaluation of insulin sensitivity and beta-cell function indexes obtained from minimal model analysis of a meal tolerance test. Diabetes. 2004; 53:1201–1207.
Article23. Mari A, Camastra S, Toschi E, Giancaterini A, Gastaldelli A, Mingrone G, et al. A model for glucose control of insulin secretion during 24 h of free living. Diabetes. 2001; 50:Suppl 1. S164–S168.
Article24. Kim DJ, Lee MS, Kim KW, Lee MK. Insulin secretory dysfunction and insulin resistance in the pathogenesis of Korean type 2 diabetes mellitus. Metabolism. 2001; 50:590–593.
Article25. Törn C. C-peptide and autoimmune markers in diabetes. Clin Lab. 2003; 49:1–10.26. Meier JJ, Menge BA, Breuer TG, Müller CA, Tannapfel A, Uhl W, et al. Functional assessment of pancreatic beta-cell area in humans. Diabetes. 2009; 58:1595–1603.27. Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006; 368:1696–1705.
Article28. Fleetcroft R, Schofield P, Ashworth M. Variations in statin prescribing for primary cardiovascular disease prevention: cross-sectional analysis. BMC Health Serv Res. 2014; 14:414.
Article29. Shim WS, Kim SK, Kim HJ, Kang ES, Ahn CW, Lim SK, et al. Decrement of postprandial insulin secretion determines the progressive nature of type-2 diabetes. Eur J Endocrinol. 2006; 155:615–622.
Article30. Saisho Y, Kou K, Tanaka K, Abe T, Kurosawa H, Shimada A, et al. Postprandial serum C-peptide to plasma glucose ratio as a predictor of subsequent insulin treatment in patients with type 2 diabetes. Endocr J. 2011; 58:315–322.
Article31. Glaser B, Cerasi E. Early intensive insulin treatment for induction of long-term glycaemic control in type 2 diabetes. Diabetes Obes Metab. 1999; 1:67–74.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Practice Guideline 2015: Oral Hypoglycemic Agents for Patients with Type 2 Diabetes
- Antihyperglycemic agent combination therapy for patients with type 2 diabetes mellitus
- A Pregnant Woman with Type 2 Diabetes Unintentionally Exposed to Metformin and Voglibose until the Second Trimester of Pregnancy: A Case Report
- Medical Therapy in Pregnant Women with Diabetes
- Clinical Characteristics of Type 2 Diabetes in Children and Adolescents