J Korean Ophthalmol Soc.
2010 May;51(5):740-745.
Glaucoma Filtering Surgery With Low Concentration of Cyclosporin A in Rabbits: A Pilot Study
- Affiliations
-
- 1Glaucoma and Cataract Services, HanGil Eye Hospital, Incheon, Korea.
- 2Department of Ophthalmology, Visual Science, St. Mary's Hospital, The Catholic University of Korea School of Medical, Seoul, Korea. jimoon@catholic.ac.kr
Abstract
- PURPOSE
The effect of 0.2% cyclosporin A (CsA) as an adjuvant therapy after glaucoma-filtering surgery was the focus of this study.
METHODS
A posterior lip sclerotomy was performed in 16 eyes of 8 rabbits, and 0.2% CsA was administered into the right eyes. The left eyes served as controls. The intraocular pressure (IOP) was measured 1, 3, 5, 7, 14, and 28 days after surgery. Hematoxylin-eosin (HE) and anti-bromodeoxyuridine (BrdU) immunocytochemical staining were performed at 1, 2, 4, and 8 weeks.
RESULTS
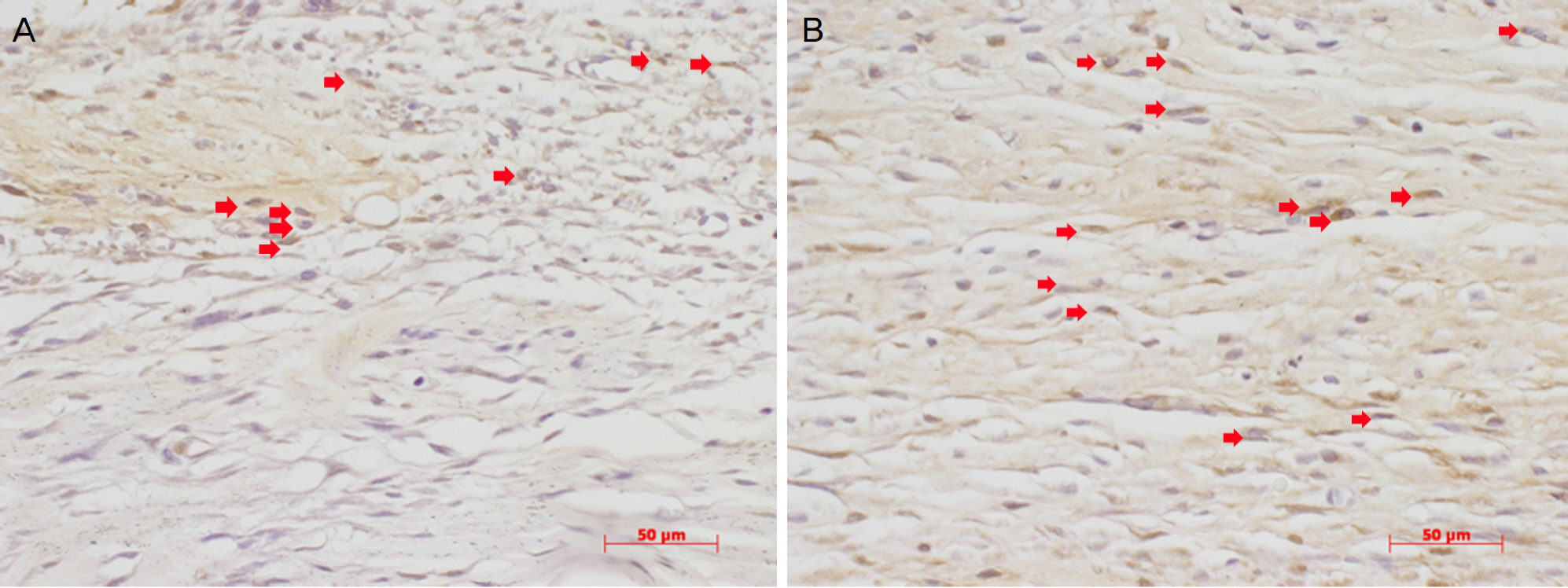
The IOP at 7 and 14 days after surgery was lower in the 0.2% CsA group and statistically significant (P=0.047, P=0.48; respectively). HE staining did not show any difference between experimental and control eyes, but anti-BrdU staining showed a lower number of positive cells in the experimental eyes at 1 week. The fibroblast proliferation rate was significantly lower 1 week after surgery in the 0.2% CsA group (P=0.003).
CONCLUSIONS
An effect of 0.2% CsA on early wound healing was observed. The data suggest that a low concentration of CsA can be useful when employed as adjuvant therapy in glaucoma filtering surgery.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Lu DW, Chang CJ, Chiang CH, et al. Wound modulation after abdominal by different formulations of antimetabolites in rabbits. J Ocul Pharmacol Ther. 2000; 16:529–38.2. Nuzzi R, Cerruti A, Finazzo C. Cyclosporine C: a study of wound-healing modulation after trabeculectomy in rabbit. Acta Ophthalmol Scand Suppl. 1998; 227:48–9.
Article3. Turaçli ME, Gündüz K, Aktan G, Sencer H. Topical cyclosporine as a possible new antimetabolite in trabeculectomy. Ophthalmic Surg Lasers. 1996; 27:438–44.
Article4. Broadway DC, Chang LP. Trabeculectomy, risk factors for failure and the preoperative state of the conjunctiva. J Glaucoma. 2001; 10:237–49.
Article5. Baek YW, Moon JI, Park CK. Effects of Taxol and Mitomycin C on Morphologic Alteration of Cultured Rabbit Subconjunctival Fibroblasts. J Korea Ophthalmol Soc. 2003; 44:202–7.6. Shouman AA, Helal A, Marzouk MA, Zaki EM. Methylcellulose, a healing inhibitor factor in an animal model of trabeculectomy. Invest Ophthalmol Vis Sci. 2006; 47:2515–9.
Article7. Lama PJ, Fechtner RD. Antifibrotics and wound healing in glaucoma surgery. Surv Ophthalmol. 2003; 48:314–46.
Article8. Park KH, Kim DM. The Effects of Topical Cyclosporin A on Glaucoma Drainage Implant Surgery in Rabbits. J Korean Ophthalmol Soc. 1995; 36:307–15.9. Garweg JG, Wegmann-Burns M, Goldblum D. Effects of abdominal, mitomycin C, azathioprine and cyclosporin A on human retinal pigmented epithelial, corneal endothelial and conjunctival cell lines. Graefes Arch Clin Exp Ophthalmol. 2006; 244:382–9.10. Bagci G, Yucel I, Duranoglu Y. The effect of cyclosporin A on abdominal rabbit subconjunctival fibroblast proliferation. Ophthalmologica. 1999; 213:114–9.11. Cristofanilli M, Pescosolido N, Risuleo G, Scarsella G. A murine cell culture model for post-trabeculectomy anfibrotic treatment: Induction of apoptosis by Cyclosporin. Acta Ophthalmol Scand. 2001; 79:309–12.
Article11. Akafo SK, Goulstine DB, Rosenthal AR. abdominal post abdominal intraocular pressures. Acta Ophthalmol (Copenh). 1992; 70:312–6.12. Mills KB. Trabeculectomy: a retrospective long-term follow-up of 444 cases. Br J Ophthalmol. 1981; 65:790–5.
Article13. Zaidi AA. Trabeculectomy: a review and 4-year follow-up. Br J Ophthalmol. 1980; 64:436–9.
Article14. Jerndal T, Lundstrom M: 330 trabeculectomies. A long time study (3–5 1/2 years). Acta Ophthalmol (Copenh). 1980; 58:947–56.15. Ahn JE, Lee YG, Hong YJ. abdominal Follow-up after Trabeculectomy with Mitomycin c. J Korea Ophthalmol Soc. 1998; 39:993–1001.16. Watson PG, Jakeman C, Ozturk M, et al. The complications of abdominal (a 20-year follow-up). Eye. 1990; 4:425–38.17. Lattanzio FA Jr, Crouch ER Jr, Mitrev PV, et al. Cyclosporin as an abdominal to glaucoma filtration surgery. J Glaucoma. 2005; 14:441–7.18. Seetner A, Morin JD. Healing of trabeculectomies in rabbits. Can J Ophthalmol. 1979; 14:121–5.19. Miller MH, Joseph NH, Ennis KW, et al. An animal model of filtration surgery. Trans Ophthalmol Soc U K. 1985; 104:893–7.20. Jampel HD, McGuigan LJ, Dunkelberger GR, et al. Cellular abdominal after experimental glaucoma filtering surgery. Arch Ophthalmol. 1988; 106:89–94.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Comparative Assessment of Filtering Bleb by Timing of subconjunctival Injection of Mitomycin-C in Glaucoma Filtering Surgery
- Low-dose 5-Fluorouracil and Glaucoma Filtering Surgery
- Filtering Operation with Adjunctive Mitomycin-C in Rabbit: Comparative Study by Subconjunctival Injection and Soaking of Mitomycin-C
- The Effect of Mitomycin on the Experimental Filtering Surgery
- 5-Fluorouracil in a Collagen Sponge and Glaucoma Filtering Surgery