Cancer Res Treat.
2018 Oct;50(4):1238-1251. 10.4143/crt.2017.534.
Prognoses and Clinical Outcomes of Primary and Recurrent Uveal Melanoma
- Affiliations
-
- 1Division of Medical Oncology, Department of Internal Medicine, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea. SSJ338@yuhs.ac
- 2College of Medicine, Yonsei University Graduate School, Seoul, Korea.
- 3Department of Pathology, Hanyang University College of Medicine, Seoul, Korea.
- 4Graduate School of Medical Science and Engineering, KAIST, Daejeon, Korea.
- 5Department of Radiation Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea.
- 6Institute of Vision Research, Department of Ophthalmology, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2424796
- DOI: http://doi.org/10.4143/crt.2017.534
Abstract
- PURPOSE
Uveal melanoma has a very poor prognosis despite successful local primary tumor treatment. In this study, we investigated prognostic factors that more accurately reflected the likelihood of recurrence and survival and delineated a prognostic model that could effectively identify different risk groups based on initial clinical parameters.
MATERIALS AND METHODS
Prognostic factors associated with distant recurrence, recurrence-free survival (RFS), progression-free survival, and overall survival from distant recurrence to death (OS2) were analyzed in 226 patients with stage I-III uveal melanoma who underwent primary local therapy.
RESULTS
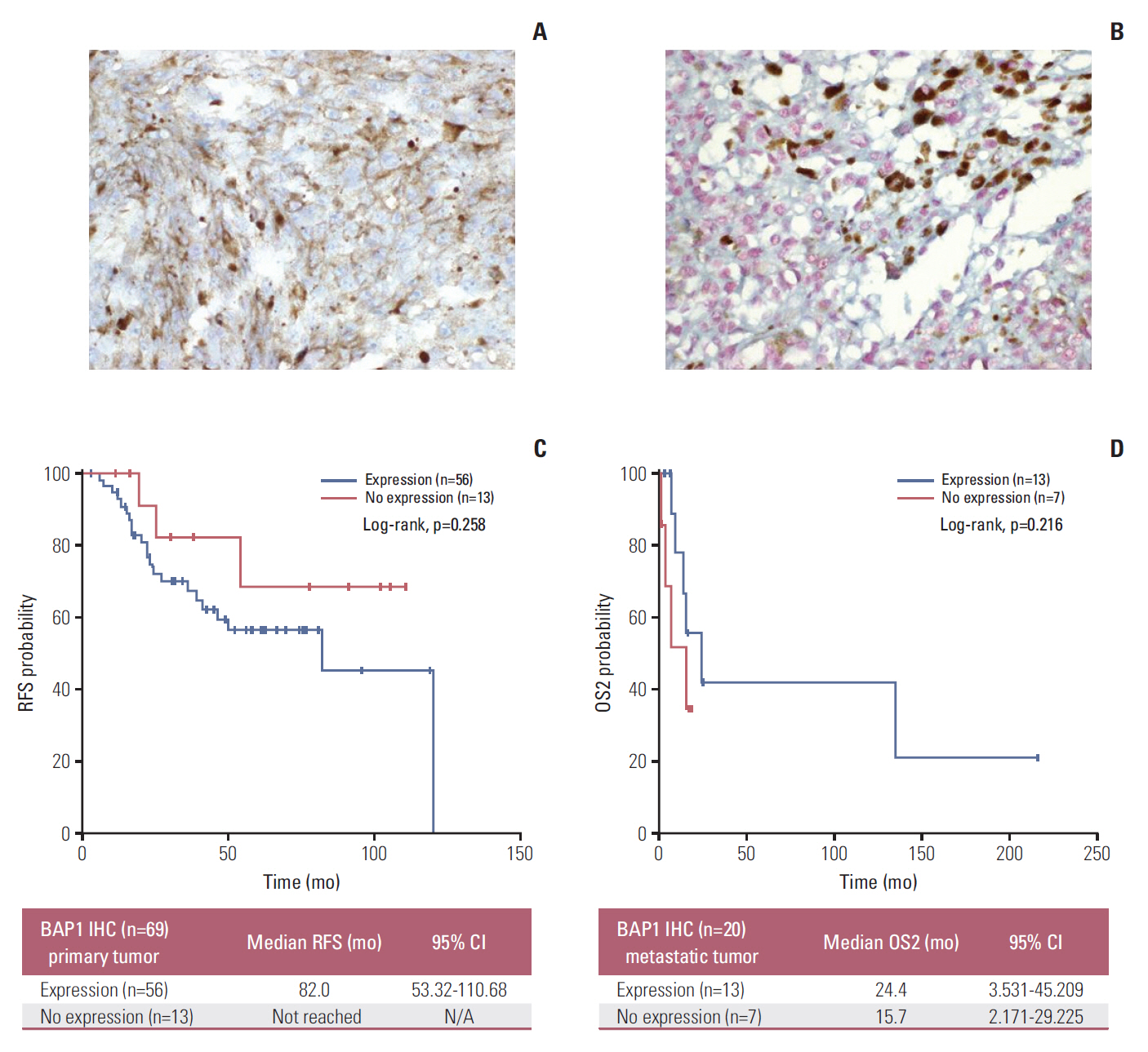
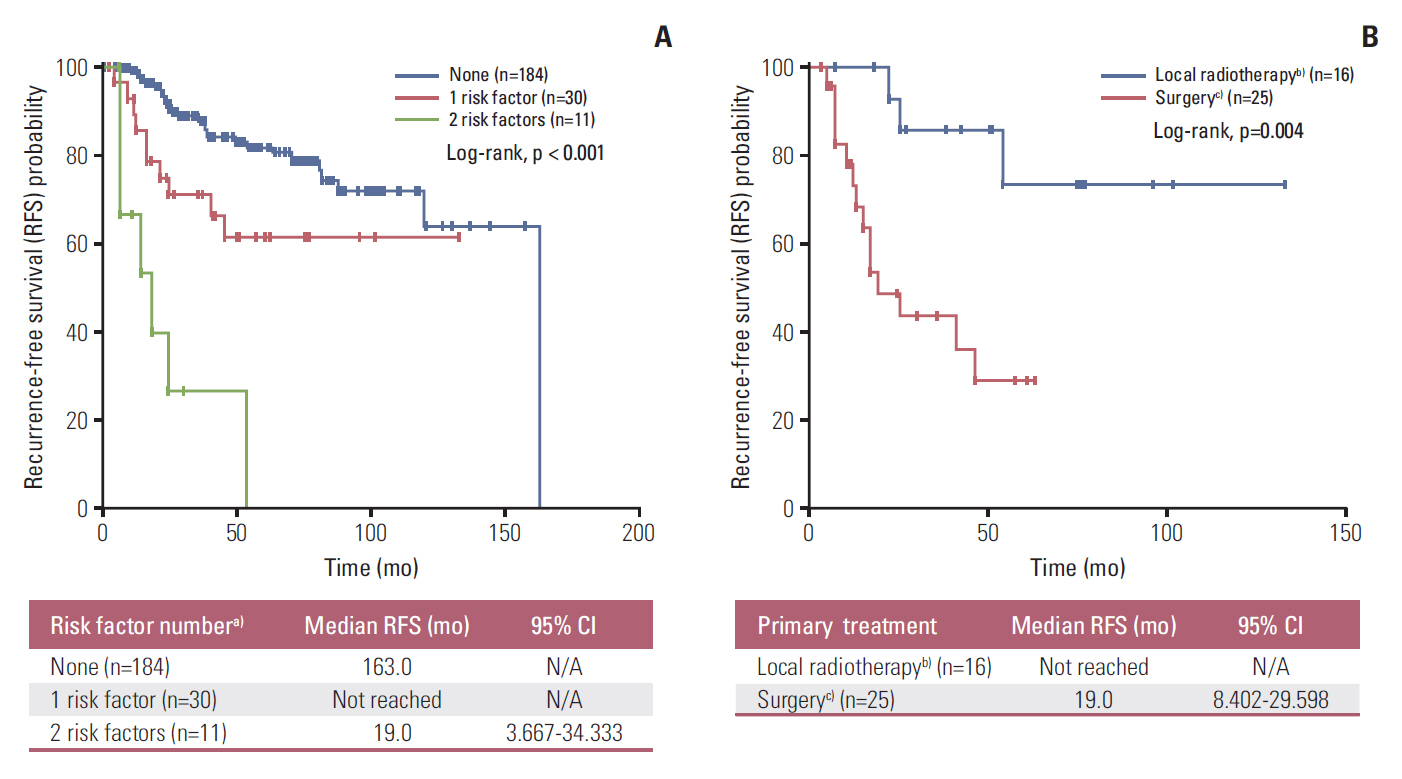
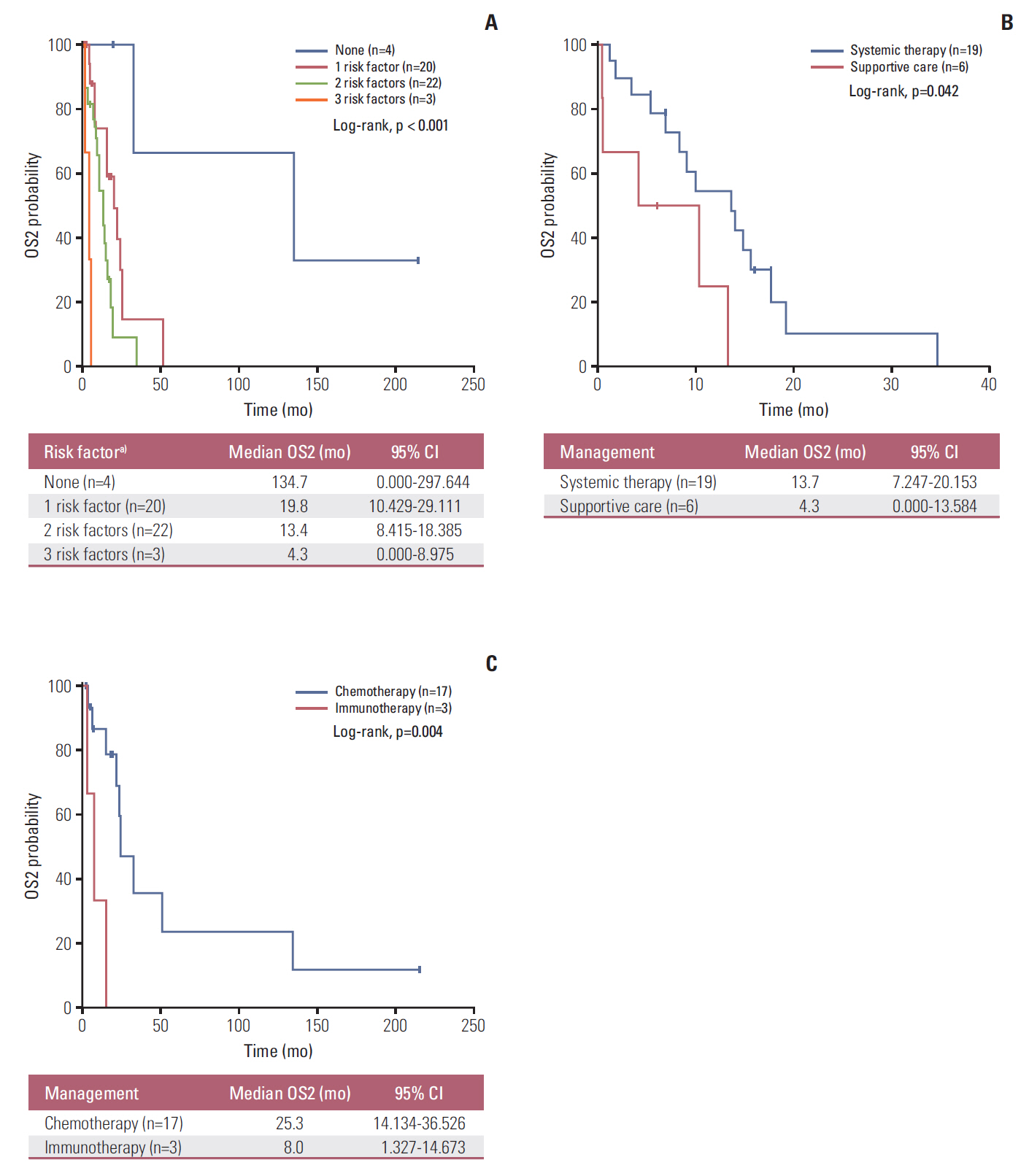
Forty-nine patients (21.7%) had distant recurrences, which occurred most frequently in the liver (87.7%). In a multivariate analysis, local radiotherapy improved RFS among patients with multiple recurrence risk factors relative to excision (not reached vs. 19.0 months, p=0.004). Patients with BRCA1-associated protein-1 (BAP1)-negative primary tumors showed a longer RFS duration after primary treatments, while those with BAP1-negative metastatic tissues had a shorter OS2 compared to those with BAP1-positive tumors, both not statistically insignificance (RFS: not reached vs. 82.0 months, p=0.258; OS2: 15.7 vs. 24.4 months, p=0.216). Male sex (hazard ratio [HR], 3.79; p=0.012), a short RFS (HR, 4.89; p=0.014), and a largest metastatic tumor linear diameter ≥ 45 mm (HR, 5.48; p=0.017) were found to correlate with worse post-recurrence survival.
CONCLUSION
Risk factors could be used to classify uveal melanoma cases and subsequently direct individual treatment strategies. Furthermore, metastasectomy appears to contribute to improved survival outcomes.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Augsburger JJ, Schneider S, Freire J, Brady LW. Survival following enucleation versus plaque radiotherapy in statistically matched subgroups of patients with choroidal melanomas: results in patients treated between 1980 and 1987. Graefes Arch Clin Exp Ophthalmol. 1999; 237:558–67.
Article2. Kujala E, Makitie T, Kivela T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003; 44:4651–9.
Article3. Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011; 118:1881–5.
Article4. Koutsandrea C, Moschos MM, Dimissianos M, Georgopoulos G, Ladas I, Apostolopoulos M. Metastasis rates and sites after treatment for choroidal melanoma by proton beam irradiation or by enucleation. Clin Ophthalmol. 2008; 2:989–95.5. Yamazaki N, Takenouchi T, Fujimoto M, Ihn H, Uchi H, Inozume T, et al. Phase 1b study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in Japanese patients with advanced melanoma (KEYNOTE-041). Cancer Chemother Pharmacol. 2017; 79:651–60.
Article6. Kottschade LA, McWilliams RR, Markovic SN, Block MS, Villasboas Bisneto J, Pham AQ, et al. The use of pembrolizumab for the treatment of metastatic uveal melanoma. Melanoma Res. 2016; 26:300–3.
Article7. Harbour JW, Onken MD, Roberson ED, Duan S, Cao L, Worley LA, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010; 330:1410–3.8. Koopmans AE, Verdijk RM, Brouwer RW, van den Bosch TP, van den Berg MM, Vaarwater J, et al. Clinical significance of immunohistochemistry for detection of BAP1 mutations in uveal melanoma. Mod Pathol. 2014; 27:1321–30.
Article9. Khattak MA, Fisher R, Hughes P, Gore M, Larkin J. Ipilimumab activity in advanced uveal melanoma. Melanoma Res. 2013; 23:79–81.
Article10. AJCC Ophthalmic Oncology Task Force. International Validation of the American Joint Committee on Cancer's 7th edition classification of uveal melanoma. JAMA Ophthalmol. 2015; 133:376–83.11. Kwon HJ, Ko JS, Kim M, Lee CS, Lee SC. Prognosis of choroidal melanoma and the result of ruthenium brachytherapy combined with transpupillary thermotherapy in Korean patients. Br J Ophthalmol. 2013; 97:653–8.
Article12. Lee JH, Lee SC, Cho A, Keum KC, Suh YG, Lee CS. Association between choroidal thickness and metabolic activity on positron emission tomography in eyes with choroidal melanoma. Am J Ophthalmol. 2015; 160:1111–5.
Article13. Damato BE, Foulds WS. Surgical resection of choroidal melanoma. In : Schachat AP, Ryan SK, editors. Retina. St. Louis, MO: Mosby;2001. p. 762–72.14. Foulds WS, Damato BE, Burton RL. Local resection versus enucleation in the management of choroidal melanoma. Eye (Lond). 1987; 1(Pt 6):676–9.
Article15. Damato B, Groenewald C, McGalliard J, Wong D. Endoresection of choroidal melanoma. Br J Ophthalmol. 1998; 82:213–8.
Article16. Buder K, Gesierich A, Gelbrich G, Goebeler M. Systemic treatment of metastatic uveal melanoma: review of literature and future perspectives. Cancer Med. 2013; 2:674–86.
Article17. Liu YC, Tsai CC, Lee FL, Lee SM, Kao SC. Mortality from uveal melanoma treated by enucleation: a 16-year survey in Taiwan. Acta Ophthalmol. 2013; 91:e583–4.18. Murali R, Wiesner T, Scolyer RA. Tumours associated with BAP1 mutations. Pathology. 2013; 45:116–26.
Article19. Rietschel P, Panageas KS, Hanlon C, Patel A, Abramson DH, Chapman PB. Variates of survival in metastatic uveal melanoma. J Clin Oncol. 2005; 23:8076–80.
Article20. Collaborative Ocular Melanoma Study Group. Assessment of metastatic disease status at death in 435 patients with large choroidal melanoma in the Collaborative Ocular Melanoma Study (COMS): COMS report no. 15. Arch Ophthalmol. 2001; 119:670–6.21. Kath R, Hayungs J, Bornfeld N, Sauerwein W, Hoffken K, Seeber S. Prognosis and treatment of disseminated uveal melanoma. Cancer. 1993; 72:2219–23.
Article22. Bedikian AY, Legha SS, Mavligit G, Carrasco CH, Khorana S, Plager C, et al. Treatment of uveal melanoma metastatic to the liver: a review of the M. D. Anderson Cancer Center experience and prognostic factors. Cancer. 1995; 76:1665–70.
Article23. Pyrhonen S. The treatment of metastatic uveal melanoma. Eur J Cancer. 1998; 34 Suppl 3:S27–30.24. Collaborative Ocular Melanoma Study Group. COMS Manual of Procedures (1999). NTIS accession No. PB95-179693. Springfield: National Technical Information Service;1999.25. Hicks C, Foss AJ, Hungerford JL. Predictive power of screening tests for metastasis in uveal melanoma. Eye (Lond). 1998; 12(Pt 6):945–8.
Article26. Gragoudas ES, Egan KM, Seddon JM, Glynn RJ, Walsh SM, Finn SM, et al. Survival of patients with metastases from uveal melanoma. Ophthalmology. 1991; 98:383–9.27. Diener-West M, Reynolds SM, Agugliaro DJ, Caldwell R, Cumming K, Earle JD, et al. Screening for metastasis from choroidal melanoma: the Collaborative Ocular Melanoma Study Group Report 23. J Clin Oncol. 2004; 22:2438–44.
Article28. Augsburger JJ, Correa ZM, Shaikh AH. Effectiveness of treatments for metastatic uveal melanoma. Am J Ophthalmol. 2009; 148:119–27.
Article29. van Essen TH, van Pelt SI, Versluis M, Bronkhorst IH, van Duinen SG, Marinkovic M, et al. Prognostic parameters in uveal melanoma and their association with BAP1 expression. Br J Ophthalmol. 2014; 98:1738–43.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Uveal Melanoma: A Review of the Literature and Personal Experiences
- Clinicopathologic features and survival outcomes of ocular melanoma: a series of 31 cases from a tertiary university hospital
- Growth and Characterization of the Uveal Melanoma in Vitro
- Epidemiologic and Clinical Features of Uveal Melanoma in Korean Patients
- Imaging Techniques for the Diagnosis of Primary Uveal Melanoma