Pediatr Gastroenterol Hepatol Nutr.
2018 Oct;21(4):329-335. 10.5223/pghn.2018.21.4.329.
Reversal of Immunogenicity in Pediatric Inflammatory Bowel Disease Patients Receiving Anti-Tumor Necrosis Factor Medications
- Affiliations
-
- 1Division of Pediatric Gastroenterology, Hepatology and Nutrition, University Hospitals/Rainbow Babies & Children's Hospital, Cleveland, OH, United States. jonathan.moses@uhhospitals.org
- KMID: 2421996
- DOI: http://doi.org/10.5223/pghn.2018.21.4.329
Abstract
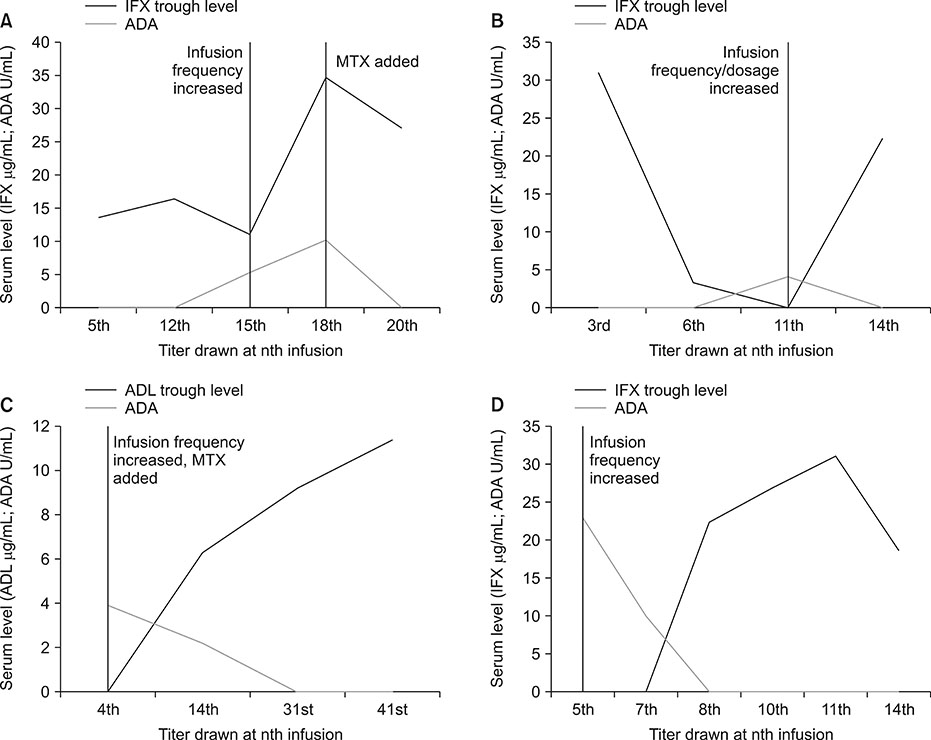
- Loss of response to anti-tumor necrosis factor (anti-TNF) agents in the treatment of inflammatory bowel disease (IBD) is a major consideration to maintain sustained response. Reversal of immunogenicity can re-establish response and increase the durability of these agents. Strategies to reverse immunogenicity include dose-intensification and/or the addition of an immunomodulator. However, there is a relative paucity of data on the efficacy of such interventions in pediatric IBD patients. Available reports have not strictly utilized homogenous mobility shift assay, which reports on anti-drug antibodies even in the presence of detectable drug, whereas prior studies have been confounded by the use of drug sensitive assays. We report four pediatric inflammatory bowel disease patients with successful reversal of immunogenicity on an anti-TNF agent using dose intensification and/or addition of an immunomodulator.
Keyword
MeSH Terms
Figure
Reference
-
1. Cuffari C, Darbari A. Inflammatory bowel disease in the pediatric and adolescent patient. Gastroenterol Clin North Am. 2002; 31:275–291.
Article2. Peyrin-Biroulet L, Lemann M. Review article: remission rates achievable by current therapies for inflammatory bowel disease. Aliment Pharmacol Ther. 2011; 33:870–879.
Article3. Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002; 359:1541–1549.
Article4. Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005; 353:2462–2476.
Article5. Cassinotti A, Travis S. Incidence and clinical significance of immunogenicity to infliximab in Crohn's disease: a critical systematic review. Inflamm Bowel Dis. 2009; 15:1264–1275.
Article6. Baert F, Noman M, Vermeire S, Van Assche G, D'Haens G, Carbonez A, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med. 2003; 348:601–608.
Article7. Hindryckx P, Novak G, Vande Casteele N, Khanna R, Laukens D, Vipul J, et al. Incidence, prevention and management of anti-drug antibodies against therapeutic antibodies in inflammatory bowel disease: a practical overview. Drugs. 2017; 77:363–377.
Article8. Minar P, Saeed SA, Afreen M, Kim MO, Denson LA. Practical use of infliximab concentration monitoring in pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2016; 62:715–722.
Article9. Wang SL, Ohrmund L, Hauenstein S, Salbato J, Reddy R, Monk P, et al. Development and validation of a homogeneous mobility shift assay for the measurement of infliximab and antibodies-to-infliximab levels in patient serum. J Immunol Methods. 2012; 382:177–188.
Article10. Gisbert JP, Panes J. Loss of response and requirement of infliximab dose intensification in Crohn's disease: a review. Am J Gastroenterol. 2009; 104:760–767.
Article11. Baert F, Kondragunta V, Lockton S, Vande Casteele N, Hauenstein S, Singh S, et al. Antibodies to adalimumab are associated with future inflammation in Crohn's patients receiving maintenance adalimumab therapy: a post hoc analysis of the Karmiris trial. Gut. 2016; 65:1126–1131.
Article12. Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): a meta-analysis. Am J Gastroenterol. 2013; 108:40–47. quiz 48.
Article13. Vande Casteele N, Gils A, Singh S, Ohrmund L, Hauenstein S, Rutgeerts P, et al. Antibody response to infliximab and its impact on pharmacokinetics can be transient. Am J Gastroenterol. 2013; 108:962–971.
Article14. Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010; 362:1383–1395.
Article15. Panaccione R, Ghosh S, Middleton S, Marquez JR, Scott BB, Flint L, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014; 146:392–400.e3.
Article16. Vande Casteele N, Khanna R, Levesque BG, Stitt L, Zou GY, Singh S, et al. The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn’s disease. Gut. 2015; 64:1539–1545.
Article17. Feuerstein JD, Nguyen GC, Kupfer SS, Falck-Ytter Y, Singh S. American Gastroenterological Association Institute Guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology. 2017; 153:827–834.
Article18. Vande Casteele N, Ferrante M, Van Assche G, Ballet V, Compernolle G, Van Steen K, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015; 148:1320–1329.e3.
Article19. Sandborn WJ, Wolf DC, Kosutic G, Parker G, Schreiber S, Lee SD, et al. Effects of transient and persistent anti-drug antibodies to certolizuman pegol: longitudinal data from a 7-year study in Crohn’s disease. Inflamm Bowel Dis. 2017; 23:1047–1056.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Biological Therapy for Inflammatory Bowel Disease in Children
- Factors Associated with the Immunogenicity of Anti-Tumor Necrosis Factor Agents in Pediatric Patients with Inflammatory Bowel Disease
- Anti-tumor Necrosis Factor Agents and Tuberculosis in Inflammatory Bowel Disease
- Clinical Aspects and Treatments for Pediatric Inflammatory Bowel Diseases
- Stem Cells in Inflammatory Bowel Disease: New Potential Therapeutic Target