Clin Exp Vaccine Res.
2016 Jul;5(2):89-100. 10.7774/cevr.2016.5.2.89.
Prospects for dengue vaccines for travelers
- Affiliations
-
- 1International Vaccine Institute, Seoul, Korea. inkyu.yoon@ivi.int
- KMID: 2421555
- DOI: http://doi.org/10.7774/cevr.2016.5.2.89
Abstract
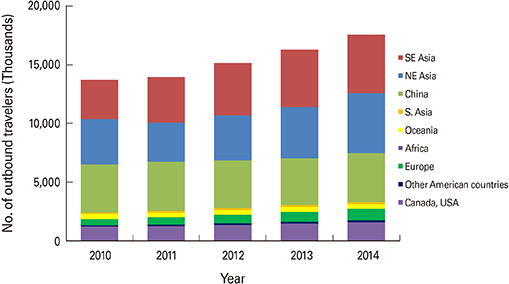
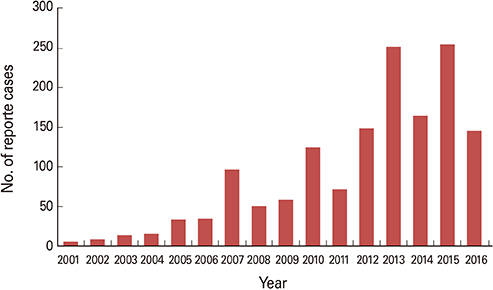
- Travel-acquired dengue cases have been increasing as the overall global dengue burden has expanded. In Korea, imported dengue cases have been reported since 2000 when it first became a notifiable disease. During the first four months of 2016, three times more dengue cases were reported in Korea than during the same period the previous year. A safe and efficacious vaccine for travelers would be beneficial to prevent dengue disease in individual travelers and potentially decrease the risk of virus spread to non-endemic areas. Here, we summarize the characteristics of dengue vaccines for travelers and review dengue vaccines currently licensed or in clinical development.
Keyword
MeSH Terms
Figure
Reference
-
1. Simmons CP, Farrar JJ, van Vinh Chau N, Wills B. Dengue. N Engl J Med. 2012; 366:1423–1432.
Article2. Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998; 11:480–496.
Article3. World Health Organization. Dengue and severe dengue [Internet]. Geneva: World Health Organization;2016. cited 2016 May 3. Available from: http://www.who.int/mediacentre/factsheets/fs117/en/.4. World Health Organization. Dengue [Internet]. Geneva: World Health Organization;2016. cited 2016 May 3. Available from: http://www.who.int/denguecontrol/en/.5. World Health Organization. Dengue, countries or areas at risk, 2013. Geneva: World Health Organization;2014.6. Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013; 496:504–507.
Article7. Brady OJ, Gething PW, Bhatt S, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012; 6:e1760.
Article8. Stanaway JD, Shepard DS, Undurraga EA, et al. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016; 16:712–723.
Article9. Suaya JA, Shepard DS, Siqueira JB, et al. Cost of dengue cases in eight countries in the Americas and Asia: a prospective study. Am J Trop Med Hyg. 2009; 80:846–855.
Article10. Shepard DS, Undurraga EA, Halasa YA, Stanaway JD. The global economic burden of dengue: a systematic analysis. Lancet Infect Dis. 2016; 04. 15. [Epub]. DOI: 10.1016/S1473-3099(16)00146-8.
Article11. Kilpatrick AM, Randolph SE. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet. 2012; 380:1946–1955.
Article12. The World Bank. International tourism, number of departures 2016 [Internet]. Washington, DC: The World Bank;2016. cited 2016 May 3. Available from: http://data.worldbank.org/indicator/ST.INT.DPRT.13. World Tourism Organization. UNWTO Tourism Highlights. 2015 edition. Madrid: World Tourism Organization;2015.14. Warne B, Weld LH, Cramer JP, et al. Travel-related infection in European travelers, EuroTravNet 2011. J Travel Med. 2014; 21:248–254.
Article15. Baaten GG, Sonder GJ, Zaaijer HL, van Gool T, Kint JA, van den Hoek A. Travel-related dengue virus infection, The Netherlands, 2006-2007. Emerg Infect Dis. 2011; 17:821–828.
Article16. Ratnam I, Black J, Leder K, et al. Incidence and seroprevalence of dengue virus infections in Australian travellers to Asia. Eur J Clin Microbiol Infect Dis. 2012; 31:1203–1210.
Article17. La Ruche G, Souares Y, Armengaud A, et al. First two autochthonous dengue virus infections in metropolitan France, September 2010. Euro Surveill. 2010; 15:19676.
Article18. Gjenero-Margan I, Aleraj B, Krajcar D, et al. Autochthonous dengue fever in Croatia, August-September 2010. Euro Surveill. 2011; 16:19805.
Article19. Anez G, Rios M. Dengue in the United States of America: a worsening scenario? Biomed Res Int. 2013; 2013:678645.20. Kutsuna S, Kato Y, Moi ML, et al. Autochthonous dengue fever, Tokyo, Japan, 2014. Emerg Infect Dis. 2015; 21:517–520.
Article21. Korea Tourism Organization. Statistics of international tourism in Korea [Internet]. Wonju: Korea Tourism Organization;2016. cited 2016 May 9. Available from: http://kto.visitkorea.or.kr/kor/notice/data/statis/profit/board/view.kto?id=426547&isNotice=false&instanceId=294&rnum=2.22. Ministry of Culture, Sports, and Tourism. Korea National Tourism Survey, 2014. Contract No. 11-1371000-000232-10. Sejong: Ministry of Culture, Sports, and Tourism;2015.23. Park JH, Lee DW. Dengue fever in South Korea, 2006-2010. Emerg Infect Dis. 2012; 18:1525–1527.
Article24. Korea Centers for Disease Control and Prevention. Disease Web Statistics System, 2016 [Internet]. Cheongju: Korea Centers for Disease Control and Prevention;2016. cited 2016 May 2. Available from: http://is.cdc.go.kr/dstat/jsp/stat/stat0001.jsp.25. Korea Centers for Disease Control and Prevention. Infectious Diseases Surveillance Yearbook, 2014. Contract No.: 11-1352159-000048-10. Cheongju: Korea Centers for Disease Control and Prevention;2015.26. Park SH, Lee MJ, Baek JH, Lee WC. Epidemiological aspects of exotic malaria and dengue fever in travelers in Korea. J Clin Med Res. 2011; 3:139–142.
Article27. Jeong YE, Lee WC, Cho JE, Han MG, Lee WJ. Comparison of the epidemiological aspects of imported Dengue cases between Korea and Japan, 2006-2010. Osong Public Health Res Perspect. 2016; 7:71–74.
Article28. Fauci AS, Morens DM. Zika virus in the Americas: yet another arbovirus threat. N Engl J Med. 2016; 374:601–604.
Article29. Gardner L, Sarkar S. A global airport-based risk model for the spread of dengue infection via the air transport network. PLoS One. 2013; 8:e72129.
Article30. Park CH, Lim H, Kim H, et al. High prevalence of Wolbachia infection in Korean populations of Aedes albopictus (Diptera: Culicidae). J Asia Pac Entomol. 2016; 19:191–194.
Article31. Korea Centers for Disease Control and Prevention. Travelers Health Information [Internet]. Cheongju: Korea Centers for Disease Control and Prevention;2016. cited 2016 May 2. Available from: http://travelinfo.cdc.go.kr/travelinfo/jsp_travelinfo/home/main/main.jsp#.32. Nasci RS, Wirtz RA, Brogdon WG. Protection against mosquitoes, ticks, and other arthropods [Internet]. Atlanta, GA: Centers for Disease Control and Prevention;2015. cited 2016 May 2. Available from: http://wwwnc.cdc.gov/travel/yellowbook/2016/the-pre-travel-consultation/protection-against-mosquitoes-ticks-other-arthropods.33. World Health Organization. Dengue control. The mosquito [Internet]. Geneva: World Health Organization;2016. cited 2016 May 13. Available from: http://www.who.int/denguecontrol/mosquito/en/.34. Alera MT, Srikiatkhachorn A, Velasco JM, et al. Incidence of dengue virus infection in adults and children in a prospective longitudinal cohort in the Philippines. PLoS Negl Trop Dis. 2016; 10:e0004337.
Article35. Wilder-Smith A, Deen JL. Dengue vaccines for travelers. Expert Rev Vaccines. 2008; 7:569–578.
Article36. Yoon IK, Getis A, Aldstadt J, et al. Fine scale spatiotemporal clustering of dengue virus transmission in children and Aedes aegypti in rural Thai villages. PLoS Negl Trop Dis. 2012; 6:e1730.
Article37. Teneza-Mora N, Lumsden J, Villasante E. A malaria vaccine for travelers and military personnel: requirements and top candidates. Vaccine. 2015; 33:7551–7558.
Article38. Benoit CM, MacLeod WB, Hamer DH, et al. Acceptability of hypothetical dengue vaccines among travelers. J Travel Med. 2013; 20:346–351.
Article39. Wilder-Smith A. Dengue vaccines for travelers: has the time come? J Travel Med. 2015; 22:200–202.
Article40. Duong V, Lambrechts L, Paul RE, et al. Asymptomatic humans transmit dengue virus to mosquitoes. Proc Natl Acad Sci U S A. 2015; 112:14688–14693.
Article41. National Travel ad Tourism Office. Profile of U.S. reside travelers visiting overseas destination: 2014 outbound. Washington, DC: U.S. Department of Commerce;2014.42. Department of Tourism of Thailand. Tourism statistics Thailand 2000-2016 [Internet]. Bangkok: Department of Tourism of Thailand;2016. cited 2016 May 16. Available from: http://www.tourism.go.th/home/listcontent/11/222/91.43. Crockett M, Keystone J. "I hate needles" and other factors impacting on travel vaccine uptake. J Travel Med. 2005; 12:Suppl 1. S41–S46.
Article44. Nothdurft HD, Dietrich M, Zuckerman JN, et al. A new accelerated vaccination schedule for rapid protection against hepatitis A and B. Vaccine. 2002; 20:1157–1162.
Article45. Rupprecht CE, Briggs D, Brown CM, et al. Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: recommendations of the advisory committee on immunization practices. MMWR Recomm Rep. 2010; 59:1–9.46. Hadinegoro SR, Arredondo-Garcia JL, Capeding MR, et al. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med. 2015; 373:1195–1206.
Article47. Smith DS. Travel medicine and vaccines for HIV-infected travelers. Top Antivir Med. 2012; 20:111–115.48. Barry M, Bia F. Pregnancy and travel. JAMA. 1989; 261:728–731.
Article49. Sabin AB, Schlesinger RW. Production of immunity to dengue with virus modified by propagation in mice. Science. 1945; 101:640–642.
Article50. Guy B, Saville M, Lang J. Development of Sanofi Pasteur tetravalent dengue vaccine. Hum Vaccin. 2010; 6.
Article51. Capeding MR, Tran NH, Hadinegoro SR, et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet. 2014; 384:1358–1365.
Article52. Villar L, Dayan GH, Arredondo-Garcia JL, et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med. 2015; 372:113–123.
Article53. Vannice KS, Durbin A, Hombach J. Status of vaccine research and development of vaccines for dengue. Vaccine. 2016; 34:2934–2938.
Article54. World Health Organization. Summary of the April 2016 meeting of the Strategic Advisory Group of Experts on immunization (SAGE). Geneva: World Health Organization;2016.55. The Philippines Department of Health. DOH releases latest dengue immunization coverage [Internet]. Manila: The Philippines Department of Health;2016. cited 2016 May 4. Available from: http://www.doh.gov.ph/node/5842.56. Chen LH, Hill DR, Wilder-Smith A. Vaccination of travelers: how far have we come and where are we going? Expert Rev Vaccines. 2011; 10:1609–1620.
Article57. Schwartz LM, Halloran ME, Durbin AP, Longini IM Jr. The dengue vaccine pipeline: implications for the future of dengue control. Vaccine. 2015; 33:3293–3298.
Article58. Durbin AP, Kirkpatrick BD, Pierce KK, Schmidt AC, Whitehead SS. Development and clinical evaluation of multiple investigational monovalent DENV vaccines to identify components for inclusion in a live attenuated tetravalent DENV vaccine. Vaccine. 2011; 29:7242–7250.
Article59. Durbin AP, Kirkpatrick BD, Pierce KK, et al. A single dose of any of four different live attenuated tetravalent dengue vaccines is safe and immunogenic in flavivirus-naive adults: a randomized, double-blind clinical trial. J Infect Dis. 2013; 207:957–965.
Article60. Kirkpatrick BD, Durbin AP, Pierce KK, et al. Robust and balanced immune responses to all 4 dengue virus serotypes following administration of a single dose of a live attenuated tetravalent Dengue vaccine to healthy, flavivirus-naive adults. J Infect Dis. 2015; 212:702–710.
Article61. Whitehead SS. Development of TV003/TV005, a single dose, highly immunogenic live attenuated dengue vaccine: what makes this vaccine different from the Sanofi-Pasteur CYD vaccine? Expert Rev Vaccines. 2016; 15:509–517.
Article62. Precioso AR, Palacios R, Thome B, Mondini G, Braga P, Kalil J. Clinical evaluation strategies for a live attenuated tetravalent dengue vaccine. Vaccine. 2015; 33:7121–7125.
Article63. Kirkpatrick BD, Whitehead SS, Pierce KK, et al. The live attenuated dengue vaccine TV003 elicits complete protection against dengue in a human challenge model. Sci Transl Med. 2016; 8:330ra36.
Article64. Osorio JE, Partidos CD, Wallace D, Stinchcomb DT. Development of a recombinant, chimeric tetravalent dengue vaccine candidate. Vaccine. 2015; 33:7112–7120.
Article65. George SL, Wong MA, Dube TJ, et al. Safety and immunogenicity of a live attenuated tetravalent Dengue vaccine candidate in flavivirus-naive adults: a randomized, double-blinded phase 1 clinical trial. J Infect Dis. 2015; 212:1032–1041.
Article66. Rupp R, Luckasen GJ, Kirstein JL, et al. Safety and immunogenicity of different doses and schedules of a live attenuated tetravalent dengue vaccine (TDV) in healthy adults: a Phase 1b randomized study. Vaccine. 2015; 33:6351–6359.
Article67. Osorio JE, Velez ID, Thomson C, et al. Safety and immunogenicity of a recombinant live attenuated tetravalent dengue vaccine (DENVax) in flavivirus-naive healthy adults in Colombia: a randomised, placebo-controlled, phase 1 study. Lancet Infect Dis. 2014; 14:830–838.
Article68. Sirivichayakul C, Barranco-Santana EA, Esquilin-Rivera I, et al. Safety and immunogenicity of a tetravalent Dengue vaccine candidate in healthy children and adults in Dengue-endemic regions: a randomized, placebo-controlled phase 2 study. J Infect Dis. 2016; 213:1562–1572.
Article69. Putnak R, Barvir DA, Burrous JM, et al. Development of a purified, inactivated, dengue-2 virus vaccine prototype in Vero cells: immunogenicity and protection in mice and rhesus monkeys. J Infect Dis. 1996; 174:1176–1184.
Article70. Yauch LE, Shresta S. Dengue virus vaccine development. Adv Virus Res. 2014; 88:315–372.
Article71. Martinez LJ, Lin L, Blaylock JM, et al. Safety and Immunogenicity of a Dengue virus serotype-1 purified-inactivated vaccine: results of a phase 1 clinical trial. Am J Trop Med Hyg. 2015; 93:454–460.
Article72. Coller BA, Clements DE, Bett AJ, Sagar SL, Ter Meulen JH. The development of recombinant subunit envelope-based vaccines to protect against dengue virus induced disease. Vaccine. 2011; 29:7267–7275.
Article73. Manoff SB, George SL, Bett AJ, et al. Preclinical and clinical development of a dengue recombinant subunit vaccine. Vaccine. 2015; 33:7126–7134.
Article74. Dengue Vaccine Initiative. Development of dengue vaccines: a review of the status and future considerations. Dengue Vaccine Initiative;2015.75. Govindarajan D, Meschino S, Guan L, et al. Preclinical development of a dengue tetravalent recombinant subunit vaccine: immunogenicity and protective efficacy in nonhuman primates. Vaccine. 2015; 33:4105–4116.
Article76. Raviprakash K, Ewing D, Simmons M, et al. Needle-free Biojector injection of a dengue virus type 1 DNA vaccine with human immunostimulatory sequences and the GM-CSF gene increases immunogenicity and protection from virus challenge in Aotus monkeys. Virology. 2003; 315:345–352.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Animal models for dengue vaccine development and testing
- Differential diagnosis of tropical diseases in travelers
- Two Cases of Dengue Fever in Family Medicine
- Epidemiological Characteristics and Risk Factors of Dengue Infection in Korean Travelers
- Risk Assessment of the Occurrence of Blood Products Infected with Dengue Virus Based on Travelers to the Areas of Dengue Outbreak