Yonsei Med J.
2018 Oct;59(8):995-1003. 10.3349/ymj.2018.59.8.995.
Development of a Novel Nonradioisotopic Assay and Cdc25B Overexpression Cell Lines for Use in Screening for Cdc25B Inhibitors
- Affiliations
-
- 1Biopharmaceutical Research Center, CJ Healthcare R&D Center, CJ HealthCare, Icheon, Korea. gyongsik.ha@cj.net
- 2Department of Biotechnology, College of Life Sciences and Biotechnology, Korea University, Seoul, Korea. cwkim@korea.ac.kr
- 3Graduate Program in Biomaterials Science and Engineering, College of Life Science and Biotechnology, Yonsei University, Seoul, Korea.
- 4Vaccine Translational Research Center, Yonsei University, Seoul, Korea.
- KMID: 2419735
- DOI: http://doi.org/10.3349/ymj.2018.59.8.995
Abstract
- PURPOSE
The cyclin-dependent kinase 1 (Cdk1) and cyclin B complex performs important roles in the transition from the G2 to M phase in the cell cycle through removal of inhibitory phosphates on Cdk1, and Cdc25B, which is a dual-specific phosphatase, mediates these dephosphorylation events. However, measuring Cdc25B activity by existing methods is hampered by inadequate nonspecific substrates and the need to use a radiolabeled isotope. The present study aimed to develop an improved method with which to properly measure Cdc25B activity using a novel nonradioisotopic assay and Cdc25B overexpression cell lines.
MATERIALS AND METHODS
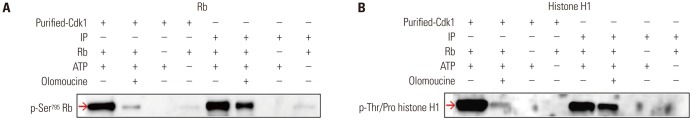
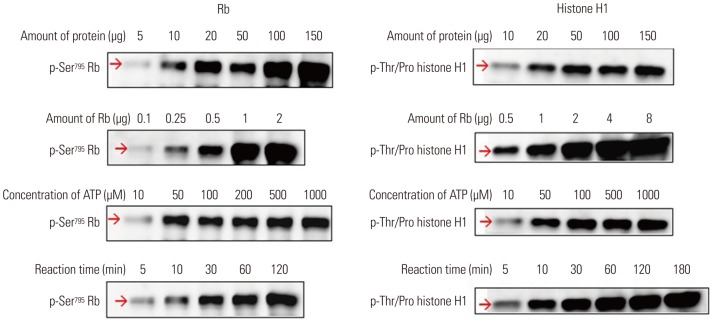
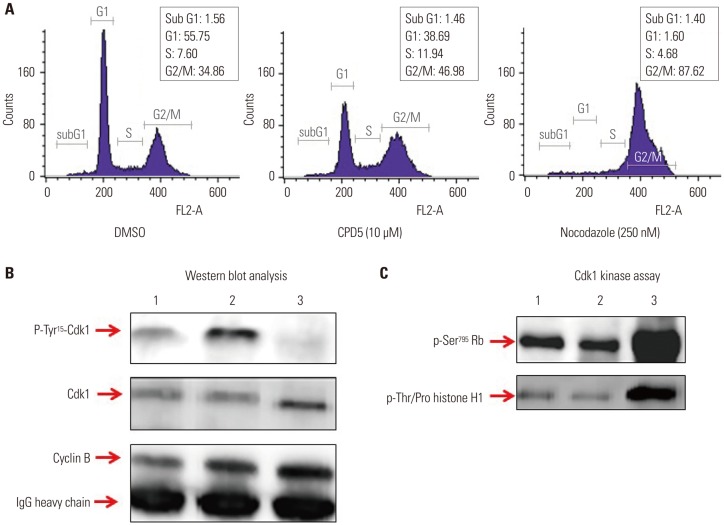
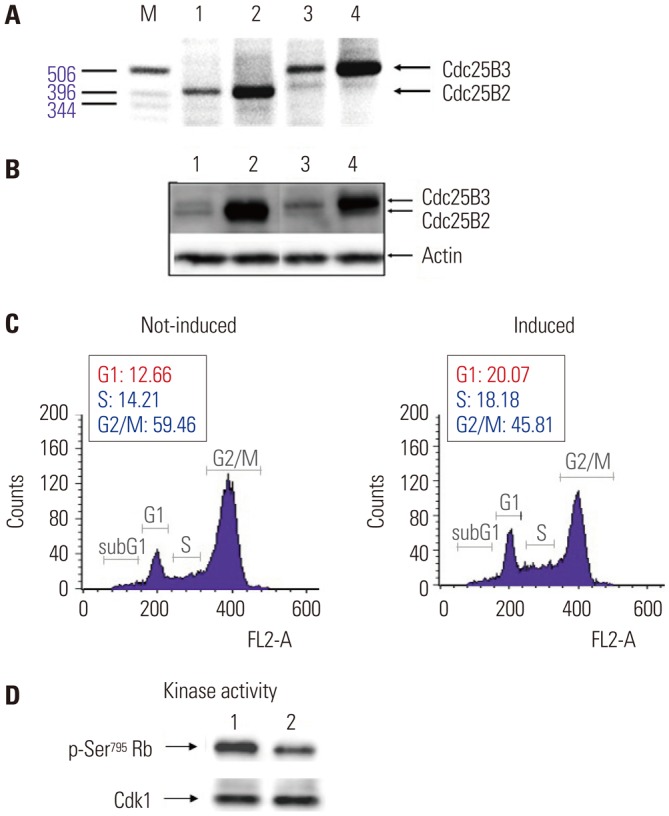
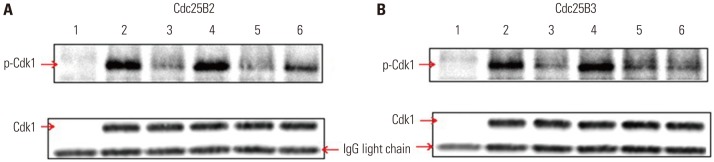
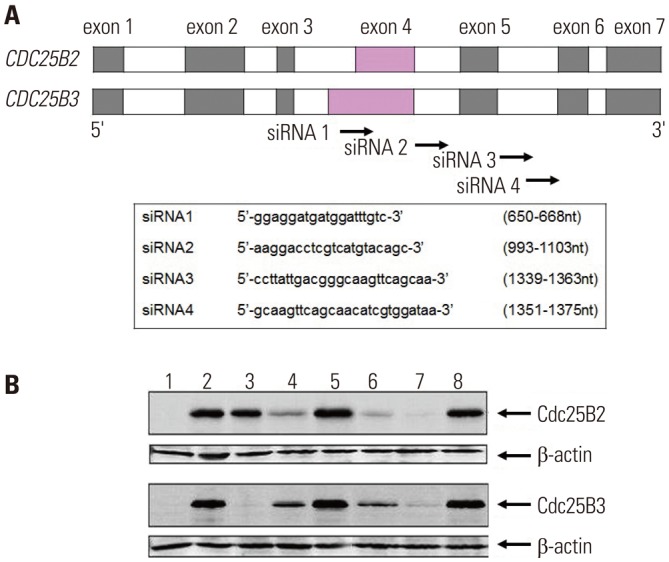
A nonradioisotopic Cdk1 kinase assay, based on Western blotting for retinoblastoma protein and histone H1, was used to analyze Cdc25B activity. Also, stable Cdc25B2 and Cdc25B3 overexpression HeLa cell lines were constructed using the tetracycline-regulated expression system and were applied as a tool for screening for inhibitors of Cdc25B.
RESULTS
The present study developed and optimized a nonradioisotopic assay method to properly measure Cdc25B activity. Furthermore, we constructed stable Cdc25B2 and Cdc25B3 overexpression HeLa cell lines for the establishment of a strong assay system with which to evaluate the specificity of Cdc25B inhibitors under conditions similar to the intracellular environment. These methods were confirmed as useful tools for measuring Cdc25B activity.
CONCLUSION
The nonradioisotopic Cdk1 kinase assay and Cdc25B overexpression cell lines developed in this study can be conveniently used as tools for screening inhibitors of Cdc25B phosphatase as anticancer drugs.
MeSH Terms
Figure
Reference
-
1. Boutros R, Lobjois V, Ducommun B. CDC25 phosphatases in cancer cells: key players? Good targets? Nat Rev Cancer. 2007; 7:495–507. PMID: 17568790.
Article2. Yu L, Orlandi L, Wang P, Orr MS, Senderowicz AM, Sausville EA, et al. UCN-01 abrogates G2 arrest through a Cdc2-dependent pathway that is associated with inactivation of the Wee1Hu kinase and activation of the Cdc25C phosphatase. J Biol Chem. 1998; 273:33455–33464. PMID: 9837924.
Article3. Sur S, Agrawal DK. Phosphatases and kinases regulating CDC25 activity in the cell cycle: clinical implications of CDC25 overexpression and potential treatment strategies. Mol Cell Biochem. 2016; 416:33–46. PMID: 27038604.
Article4. Gabrielli BG, De Souza CP, Tonks ID, Clark JM, Hayward NK, Ellem KA. Cytoplasmic accumulation of cdc25B phosphatase in mitosis triggers centrosomal microtubule nucleation in HeLa cells. J Cell Sci. 1996; 109(Pt 5):1081–1093. PMID: 8743955.
Article5. Lammer C, Wagerer S, Saffrich R, Mertens D, Ansorge W, Hoffmann I. The cdc25B phosphatase is essential for the G2/M phase transition in human cells. J Cell Sci. 1998; 111(Pt 16):2445–2453. PMID: 9683638.
Article6. Galaktionov K, Lee AK, Eckstein J, Draetta G, Meckler J, Loda M, et al. CDC25 phosphatases as potential human oncogenes. Science. 1995; 269:1575–1577. PMID: 7667636.
Article7. Takemasa I, Yamamoto H, Sekimoto M, Ohue M, Noura S, Miyake Y, et al. Overexpression of CDC25B phosphatase as a novel marker of poor prognosis of human colorectal carcinoma. Cancer Res. 2000; 60:3043–3050. PMID: 10850455.8. Honda R, Ohba Y, Nagata A, Okayama H, Yasuda H. Dephosphorylation of human p34cdc2 kinase on both Thr-14 and Tyr-15 by human cdc25B phosphatase. FEBS Lett. 1993; 318:331–334. PMID: 8440392.9. Gottlin EB, Xu X, Epstein DM, Burke SP, Eckstein JW, Ballou DP, et al. Kinetic analysis of the catalytic domain of human cdc25B. J Biol Chem. 1996; 271:27445–27449. PMID: 8910325.
Article10. McCain DF, Catrina IE, Hengge AC, Zhang ZY. The catalytic mechanism of Cdc25A phosphatase. J Biol Chem. 2002; 277:11190–11200. PMID: 11805096.
Article11. Parks JM, Hu H, Rudolph J, Yang W. Mechanism of Cdc25B phosphatase with the small molecule substrate p-nitrophenyl phosphate from QM/MM-MFEP calculations. J Phys Chem B. 2009; 113:5217–5224. PMID: 19301836.
Article12. Tamura K, Southwick EC, Kerns J, Rosi K, Carr BI, Wilcox C, et al. Cdc25 inhibition and cell cycle arrest by a synthetic thioalkyl vitamin K analogue. Cancer Res. 2000; 60:1317–1325. PMID: 10728693.13. Hoffmann I, Clarke PR, Marcote MJ, Karsenti E, Draetta G. Phosphorylation and activation of human cdc25-C by cdc2--cyclin B and its involvement in the self-amplification of MPF at mitosis. EMBO J. 1993; 12:53–63. PMID: 8428594.
Article14. Blomberg I, Hoffmann I. Ectopic expression of Cdc25A accelerates the G(1)/S transition and leads to premature activation of cyclin E- and cyclin A-dependent kinases. Mol Cell Biol. 1999; 19:6183–6194. PMID: 10454565.15. Rubin SM. Deciphering the retinoblastoma protein phosphorylation code. Trends Biochem Sci. 2013; 38:12–19. PMID: 23218751.
Article16. Blewett A. Cell division: CDK1 in the driving seat. Nat Rev Mol Cell Biol. 2007; 8:755–756.17. Cossy J, Belotti D, Brisson M, Skoko JJ, Wipf P, Lazo JS. Biological evaluation of newly synthesized quinoline-5,8-quinones as Cdc25B inhibitors. Bioorg Med Chem. 2006; 14:6283–6287. PMID: 16782352.
Article18. Forrest AR, McCormack AK, DeSouza CP, Sinnamon JM, Tonks ID, Hayward NK, et al. Multiple splicing variants of cdc25B regulate G2/M progression. Biochem Biophys Res Commun. 1999; 260:510–515. PMID: 10403798.
Article19. Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press;1989.20. Morgan DL. The cell cycle: principles of control. London: New Science Press;2007.21. Münger K, Howley PM. Human papillomavirus immortalization and transformation functions. Virus Res. 2002; 89:213–228. PMID: 12445661.
Article22. Freedman BS, Heald R. Functional comparison of H1 histones in Xenopus reveals isoform-specific regulation by Cdk1 and RanGTP. Curr Biol. 2010; 20:1048–1052. PMID: 20471264.
Article23. Talvinen K, Tuikkala J, Grönroos J, Huhtinen H, Kronqvist P, Aittokallio T, et al. Biochemical and clinical approaches in evaluating the prognosis of colon cancer. Anticancer Res. 2006; 26:4745–4751. PMID: 17214335.24. Hernández S, Hernández L, Bea S, Pinyol M, Nayach I, Bellosillo B, et al. cdc25a and the splicing variant cdc25b2, but not cdc25B1, -B3 or -C, are over-expressed in aggressive human non-Hodgkin's lymphomas. Int J Cancer. 2000; 89:148–152. PMID: 10754492.
Article25. Garzon RJ, Zehner ZE. Multiple silencer elements are involved in regulating the chicken vimentin gene. Mol Cell Biol. 1994; 14:934–943. PMID: 8289833.
Article26. Leader M, Collins M, Patel J, Henry K. Vimentin: an evaluation of its role as a tumour marker. Histopathology. 1987; 11:63–72. PMID: 2435649.
Article27. Bomsztyk K, Denisenko O, Ostrowski J. hnRNP K: one protein multiple processes. Bioessays. 2004; 26:629–638. PMID: 15170860.
Article28. Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005; 62:670–684. PMID: 15770419.
Article29. Wang X, Wang Q, Lin H, Li S, Sun L, Yang Y. HSP72 and gp96 in gastroenterological cancers. Clin Chim Acta. 2013; 417:73–79. PMID: 23266770.
Article30. Song Y, Luo Q, Long H, Hu Z, Que T, Zhang X, et al. Alpha-enolase as a potential cancer prognostic marker promotes cell growth, migration, and invasion in glioma. Mol Cancer. 2014; 13:65. PMID: 24650096.
Article31. Zhou W, Capello M, Fredolini C, Piemonti L, Liotta LA, Novelli F, et al. Mass spectrometry analysis of the post-translational modifications of alpha-enolase from pancreatic ductal adenocarcinoma cells. J Proteome Res. 2010; 9:2929–2936. PMID: 20433201.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Cdc25A, Cdc25B and Cdc25C in cervical carcinoma
- Cell Adhcsion and Carbohydrate Structure of Colon Cancer Cell Line KM12C and Subpopulation
- Comparative Measurement of FVIII Inhibitors in Hemophilia A Patients Using ELISA and the Bethesda Assay
- Effects of p27 Overexpression on Head and Neck Squamous Cell Carcinoma Cell Lines
- Celecoxib and Indomethacin Inhibit the Growth of Cancer and Oral Keratinocyte Cell Lines via Cyclooxygenase-2 Independent Mechanism