Yonsei Med J.
2017 Jul;58(4):800-806. 10.3349/ymj.2017.58.4.800.
Clinical Efficacy and Safety of Naftopidil Treatment for Patients with Benign Prostatic Hyperplasia and Hypertension: A Prospective, Open-Label Study
- Affiliations
-
- 1Department of Urology, Catholic Kwandong University, International St. Mary's Hospital, Incheon, Korea.
- 2Department of Urology, Urological Science Institute, Yonsei University College of Medicine, Seoul, Korea. leeseh@yuhs.ac
- KMID: 2419087
- DOI: http://doi.org/10.3349/ymj.2017.58.4.800
Abstract
- PURPOSE
To investigate the efficacy and safety of naftopidil for benign prostatic hyperplasia (BPH) patients, mainly focusing on changes in blood pressure (BP).
MATERIALS AND METHODS
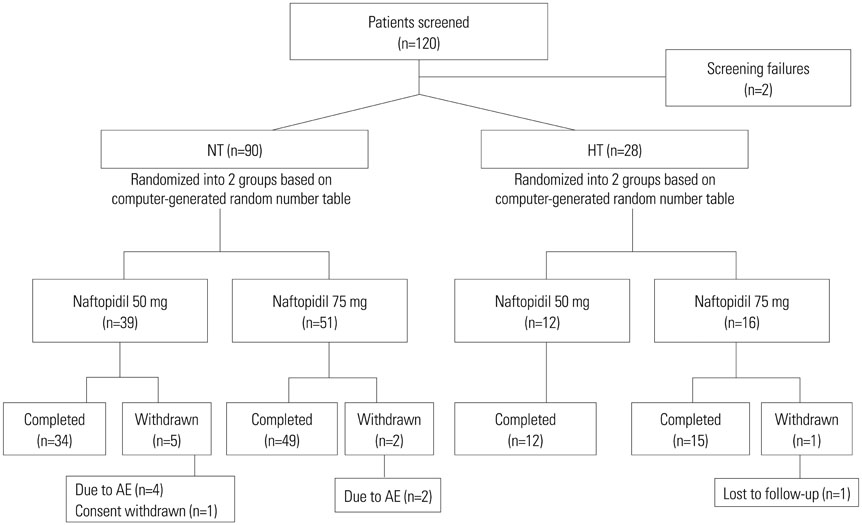
Of a total of 118 patients, 90 normotensive (NT) and 28 hypertensive (HT) patients were randomly assigned to be treated with naftopidil 50 mg or 75 mg for 12 weeks, once-daily. Safety and efficacy were assessed by analyzing changes from baseline in systolic/diastolic BP and total International Prostate Symptom Score (IPSS) at 4 and 12 weeks. Adverse events (AEs), obstructive/irritative subscores, quality of life (QoL) score, maximum urinary flow rate (Qmax), and benefit, satisfaction with treatment, and willingness to continue treatment (BSW) questionnaire were also analyzed.
RESULTS
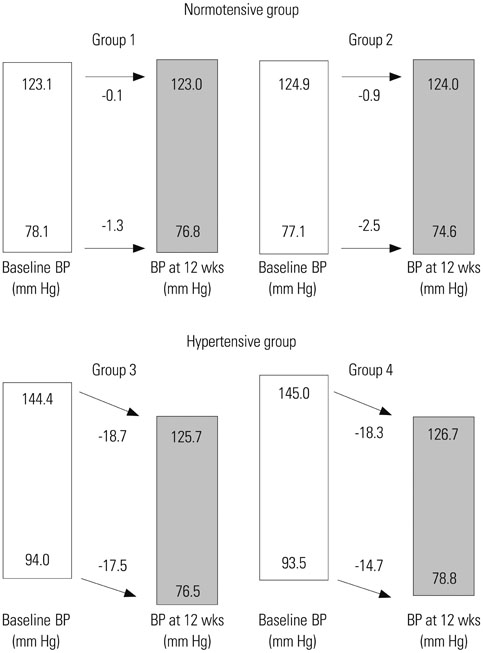
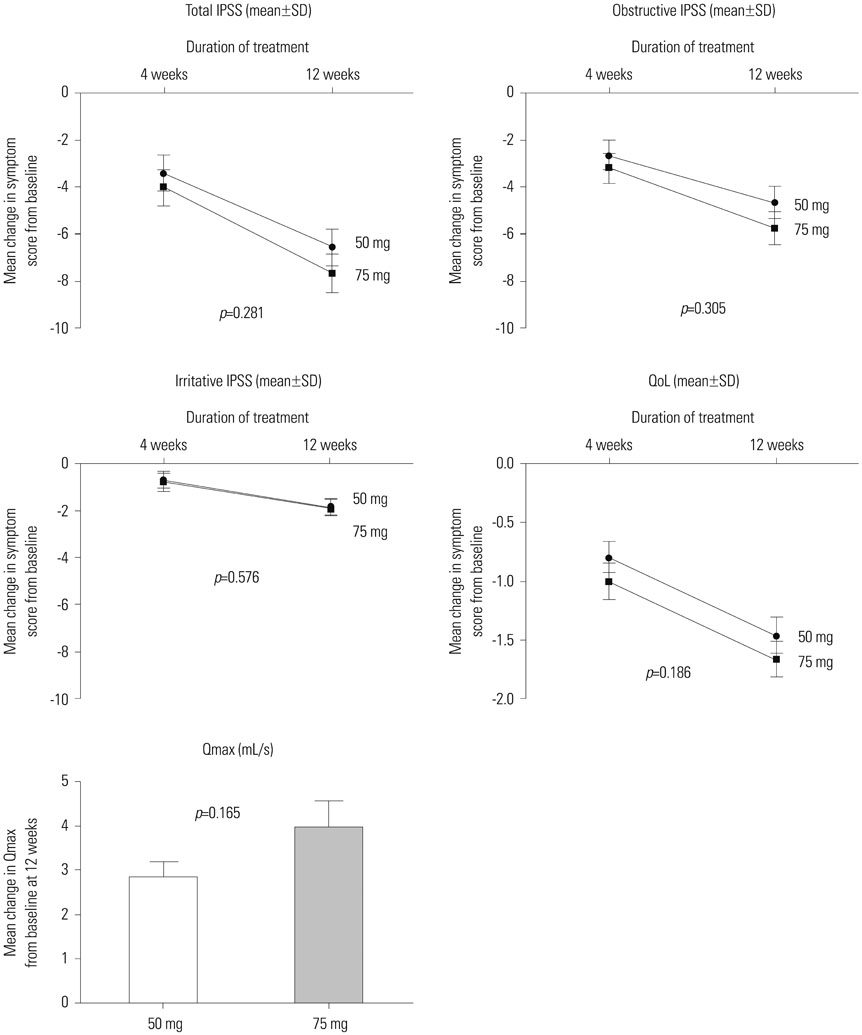
Naftopidil treatment decreased mean systolic BP by 18.7 mm Hg for the HT 50 mg group (p<0.001) and by 18.3 mm Hg for the HT 75 mg group (p<0.001) and mean diastolic BP by 17.5 mm Hg for the HT 50 mg group (p<0.001) and by 14.7 mm Hg for the HT 75 mg group (p=0.022). In the NT groups (both naftopidil 50 mg and 75 mg), naftopidil elicited no significant changes in BP from baseline values. After 12 weeks, naftopidil 50 and 75 mg groups showed significant improvements in IPSS scores (total, obstructive/irritative subscores, QoL score) and Qmax from baseline. AEs were reported in 7.8% (50 mg group) and 2.9% (75 mg group) of patients. In both the 50 mg and 75 mg groups, >86% of all patients agreed to continue their current medications.
CONCLUSION
Our results suggest that naftopidil treatment in BPH patients with hypertension allows for optimal management of BP within the normal range.
Keyword
MeSH Terms
-
Adrenergic alpha-Antagonists/adverse effects/pharmacology/therapeutic use
Aged
Blood Pressure/drug effects
Demography
Humans
Hypertension/*complications/*drug therapy
Male
Middle Aged
Naphthalenes/*adverse effects/pharmacology/*therapeutic use
Patient Compliance
Patient Satisfaction
Piperazines/*adverse effects/pharmacology/*therapeutic use
Prospective Studies
Prostatic Hyperplasia/*complications/*drug therapy
Quality of Life
Surveys and Questionnaires
Treatment Outcome
Adrenergic alpha-Antagonists
Naphthalenes
Piperazines
Figure
Reference
-
1. Boyle P, Napalkov P. The epidemiology of benign prostatic hyperplasia and observations on concomitant hypertension. Scand J Urol Nephrol Suppl. 1995; 168:7–12.2. Parsons JK. Modifiable risk factors for benign prostatic hyperplasia and lower urinary tract symptoms: new approaches to old problems. J Urol. 2007; 178:395–401.
Article3. Lee SH, Park KK, Mah SY, Chung BH. Effects of α-blocker ‘add on’ treatment on blood pressure in symptomatic BPH with or without concomitant hypertension. Prostate Cancer Prostatic Dis. 2010; 13:333–337.
Article4. Fawzy A, Hendry A, Cook E, Gonzalez F. Long-term (4 year) efficacy and tolerability of doxazosin for the treatment of concurrent benign prostatic hyperplasia and hypertension. Int J Urol. 1999; 6:346–354.
Article5. Chung BH, Hong SJ. Long-term follow-up study to evaluate the efficacy and safety of the doxazosin gastrointestinal therapeutic system in patients with benign prostatic hyperplasia with or without concomitant hypertension. BJU Int. 2006; 97:90–95.
Article6. McConnell JD, Roehrborn CG, Bautista OM, Andriole GL Jr, Dixon CM, Kusek JW, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003; 349:2387–2398.
Article7. Kirby RS, Roehrborn C, Boyle P, Bartsch G, Jardin A, Cary MM, et al. Efficacy and tolerability of doxazosin and finasteride, alone or in combination, in treatment of symptomatic benign prostatic hyperplasia: the Prospective European Doxazosin and Combination Therapy (PREDICT) trial. Urology. 2003; 61:119–126.
Article8. Takei R, Ikegaki I, Shibata K, Tsujimoto G, Asano T. Naftopidil, a novel alpha1-adrenoceptor antagonist, displays selective inhibition of canine prostatic pressure and high affinity binding to cloned human alpha1-adrenoceptors. Jpn J Pharmacol. 1999; 79:447–454.9. Gotoh M, Kamihira O, Kinukawa T, Ono Y, Ohshima S, Origasa H. Tokai Urological Clinical Trial Group. Comparison of tamsulosin and naftopidil for efficacy and safety in the treatment of benign prostatic hyperplasia: a randomized controlled trial. BJU Int. 2005; 96:581–586.
Article10. Nishino Y, Masue T, Miwa K, Takahashi Y, Ishihara S, Deguchi T. Comparison of two alpha1-adrenoceptor antagonists, naftopidil and tamsulosin hydrochloride, in the treatment of lower urinary tract symptoms with benign prostatic hyperplasia: a randomized crossover study. BJU Int. 2006; 97:747–751.
Article11. Perumal C, Chowdhury PS, Ananthakrishnan N, Nayak P, Gurumurthy S. A comparison of the efficacy of naftopidil and tamsulosin hydrochloride in medical treatment of benign prostatic enlargement. Urol Ann. 2015; 7:74–78.
Article12. Komiya A, Suzuki H, Awa Y, Egoshi K, Onishi T, Nakatsu H, et al. Clinical effect of naftopidil on the quality of life of patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a prospective study. Int J Urol. 2010; 17:555–562.
Article13. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003; 42:1206–1252.
Article14. Cifkova R, Erdine S, Fagard R, Farsang C, Heagerty AM, Kiowski W, et al. Practice guidelines for primary care physicians: 2003 ESH/ESC hypertension guidelines. J Hypertens. 2003; 21:1779–1786.15. Kirby RS. Doxazosin in benign prostatic hyperplasia: effects on blood pressure and urinary flow in normotensive and hypertensive men. Urology. 1995; 46:182–186.
Article16. Kaplan SA, Meade-D'Alisera P, Quiñones S, Soldo KA. Doxazosin in physiologically and pharmacologically normotensive men with benign prostatic hyperplasia. Urology. 1995; 46:512–517.
Article17. Himmel HM, Glossmann H, Ravens U. Naftopidil, a new alpha-adrenoceptor blocking agent with calcium antagonistic properties: characterization of Ca2+ antagonistic effects. J Cardiovasc Pharmacol. 1991; 17:213–221.
Article18. Sponer G, Borbe HO, Müller-Beckmann B, Freud P, Jakob B. Naftopidil, a new adrenoceptor blocking agent with Ca(2+)-antagonistic properties: interaction with adrenoceptors. J Cardiovasc Pharmacol. 1992; 20:1006–1013.
Article19. Grundke M, Himmel HM, Wettwer E, Borbe HO, Ravens U. Characterization of Ca(2+)-antagonistic effects of three metabolites of the new antihypertensive agent naftopidil, (naphthyl)hydroxy-naftopidil, (phenyl)hydroxy-naftopidil, and O-desmethyl-naftopidil. J Cardiovasc Pharmacol. 1991; 18:918–925.
Article20. Sugaya K, Nishijima S, Miyazato M, Ashitomi K, Hatano T, Ogawa Y. Effects of intrathecal injection of tamsulosin and naftopidil, alpha-1A and -1D adrenergic receptor antagonists, on bladder activity in rats. Neurosci Lett. 2002; 328:74–76.
Article21. Utsunomiya N, Matsumoto K, Tsunemori H, Muguruma K, Kawakita M, Kamiyama Y, et al. [A crossover comparison study on lower urinary tract symptoms with overactive bladder secondary to benign prostatic hyperplasia: naftopidil versus tamsulosin with solifenacin]. Hinyokika Kiyo. 2016; 62:341–347.22. Sakai H, Igawa T, Onita T, Furukawa M, Hakariya T, Hayashi M, et al. [Efficacy of naftopidil in patients with overactive bladder associated with benign prostatic hyperplasia: prospective randomized controlled study to compare differences in efficacy between morning and evening medication]. Hinyokika Kiyo. 2011; 57:7–13.23. Oh-oka H. Effect of naftopidil on nocturia after failure of tamsulosin. Urology. 2008; 72:1051–1055.
Article24. Hampel C, Dolber PC, Smith MP, Savic SL, Th roff JW, Thor KB, et al. Modulation of bladder alpha1-adrenergic receptor subtype expression by bladder outlet obstruction. J Urol. 2002; 167:1513–1521.
Article25. Schwinn DA. The role of alpha1-adrenergic receptor subtypes in lower urinary tract symptoms. BJU Int. 2001; 88:Suppl 2. 27–34.
Article26. Malloy BJ, Price DT, Price RR, Bienstock AM, Dole MK, Funk BL, et al. Alpha1-adrenergic receptor subtypes in human detrusor. J Urol. 1998; 160(3 Pt 1):937–943.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Abdominal Obesity on the Increased Prevalence Rate of Hypertension and Diabetes Mellitus in Benign Prostatic Hyperplasia Patients
- Long-Term Efficacy and Safety of Terazosin in the Symptomatic Treatment of Benign Prostatic Hyperplasia
- The Experience with Combination of Finasteride and Tamsulosin on Benign Prostatic Hyperplasia
- A Prominently Large Glans penis as a Possible sign of Benign Prostatic Hyperplasia
- Technical Aspects of Holmium Laser Enucleation of the Prostate for Benign Prostatic Hyperplasia