Ewha Med J.
2018 Jul;41(3):63-74. 10.12771/emj.2018.41.3.63.
Expression of CD44 according to Clinicopathologic Characteristics of Gastric Cancer
- Affiliations
-
- 1Department of Internal Medicine, Ewha Womans University College of Medicine, Seoul, Korea. mooncm27@ewha.ac.kr
- 2Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- 3Tissue Injury Defense Research Center, Ewha Womans University, Seoul, Korea.
- 4Department of Pathology, Ewha Womans University College of Medicine, Seoul, Korea.
- KMID: 2417943
- DOI: http://doi.org/10.12771/emj.2018.41.3.63
Abstract
OBJECTIVES
Cancer stem cells are defined as focal cluster of cells within a tumor that possess the capacity for self-renewal and differentiation into phenotypically heterogeneous cells. Cluster of differentiation 44 (CD44) is considered one of the gastric cancer stem cell markers. We aimed to investigate how the expression of CD44 varies according to the clinicopathologic characteristics in gastric cancer.
METHODS
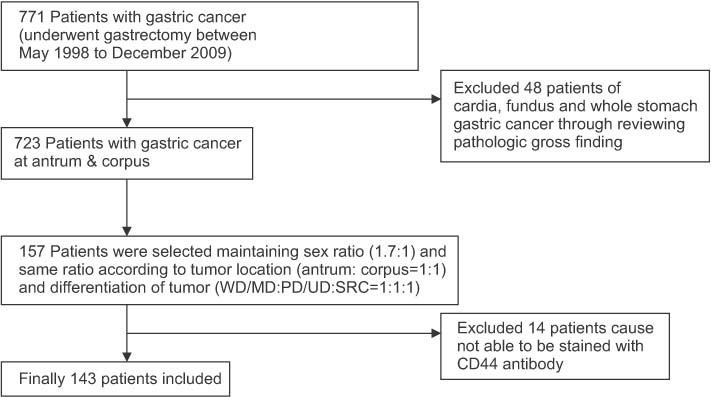
For this study, 157 patients who received an operation due to gastric cancer between May 1998 and December 2009 were selected. CD44 immunohistochemistry was reviewed using the semi-quantitative scoring of intensity and proportion. The sum of the intensity and proportion scores was calculated, and a score of 2 or less was deemed "˜CD44-negative' and 3 or more as "˜CD44-positive.'
RESULTS
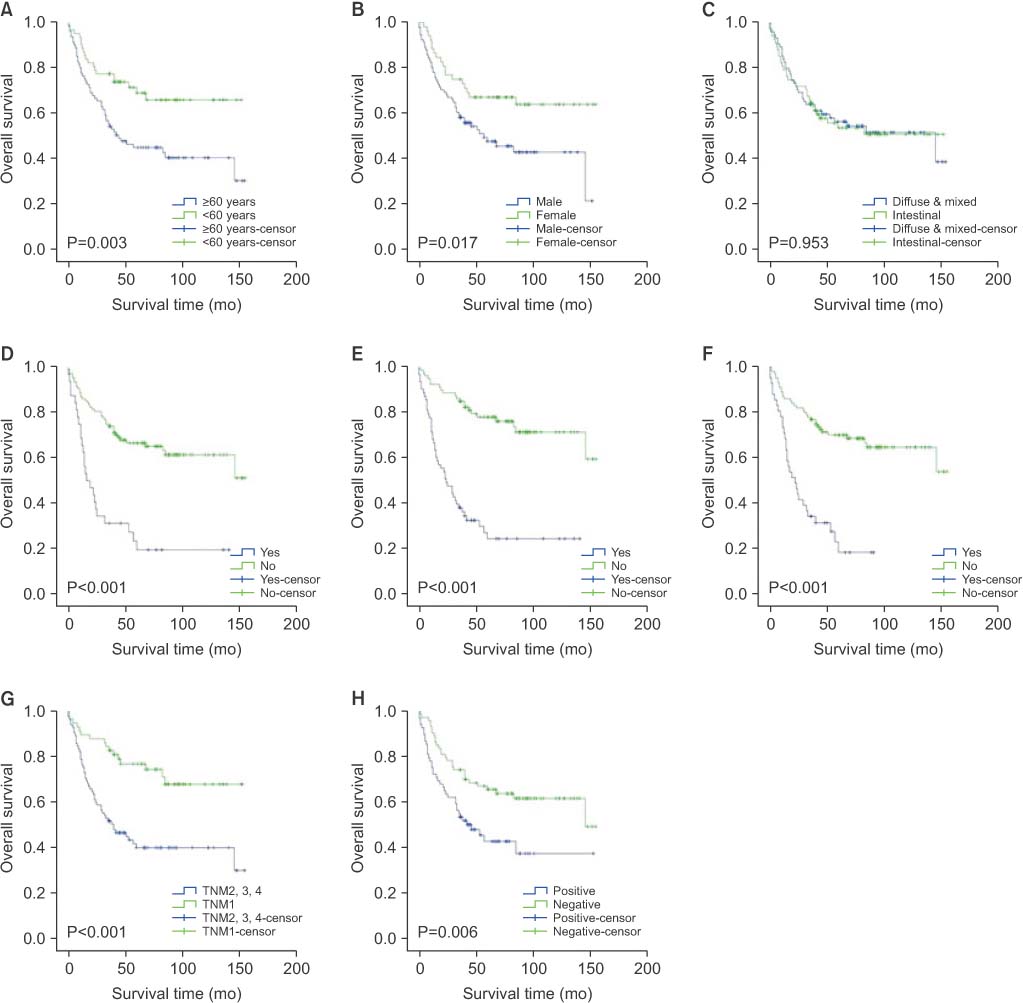
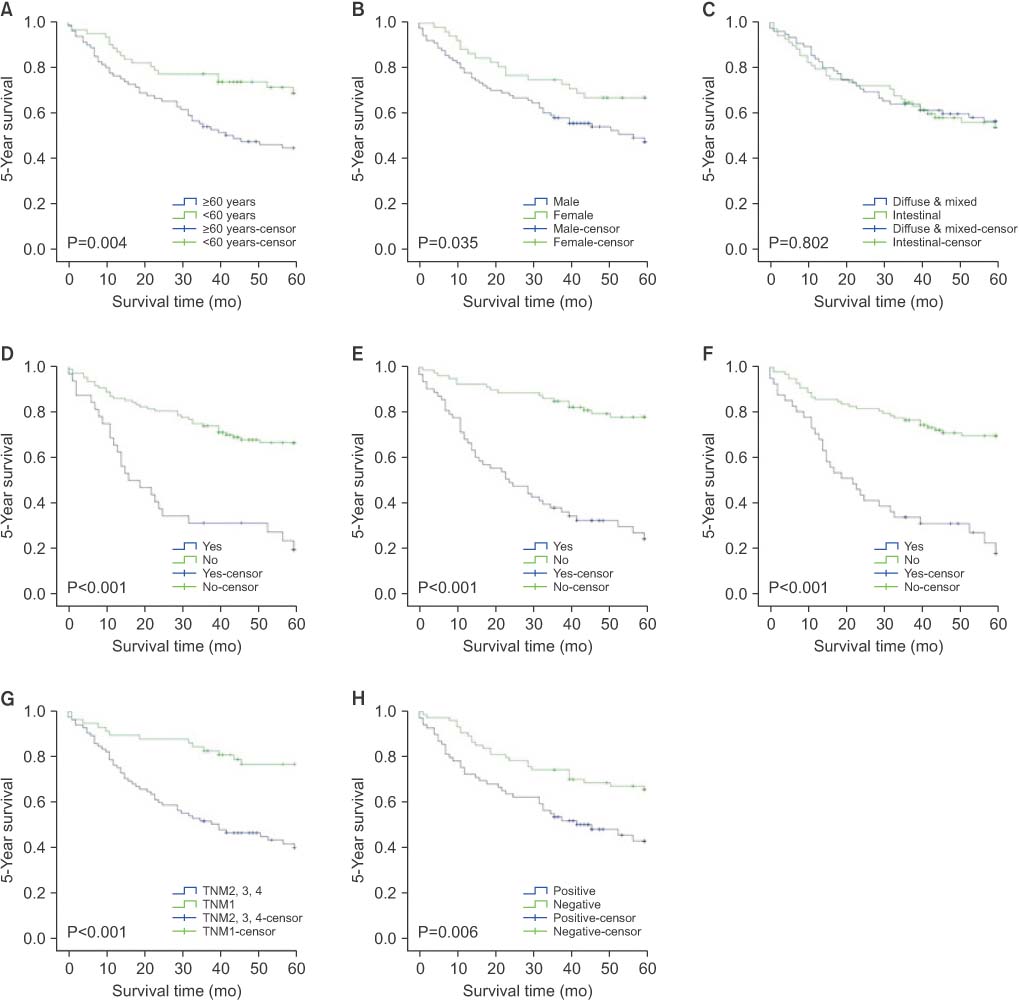
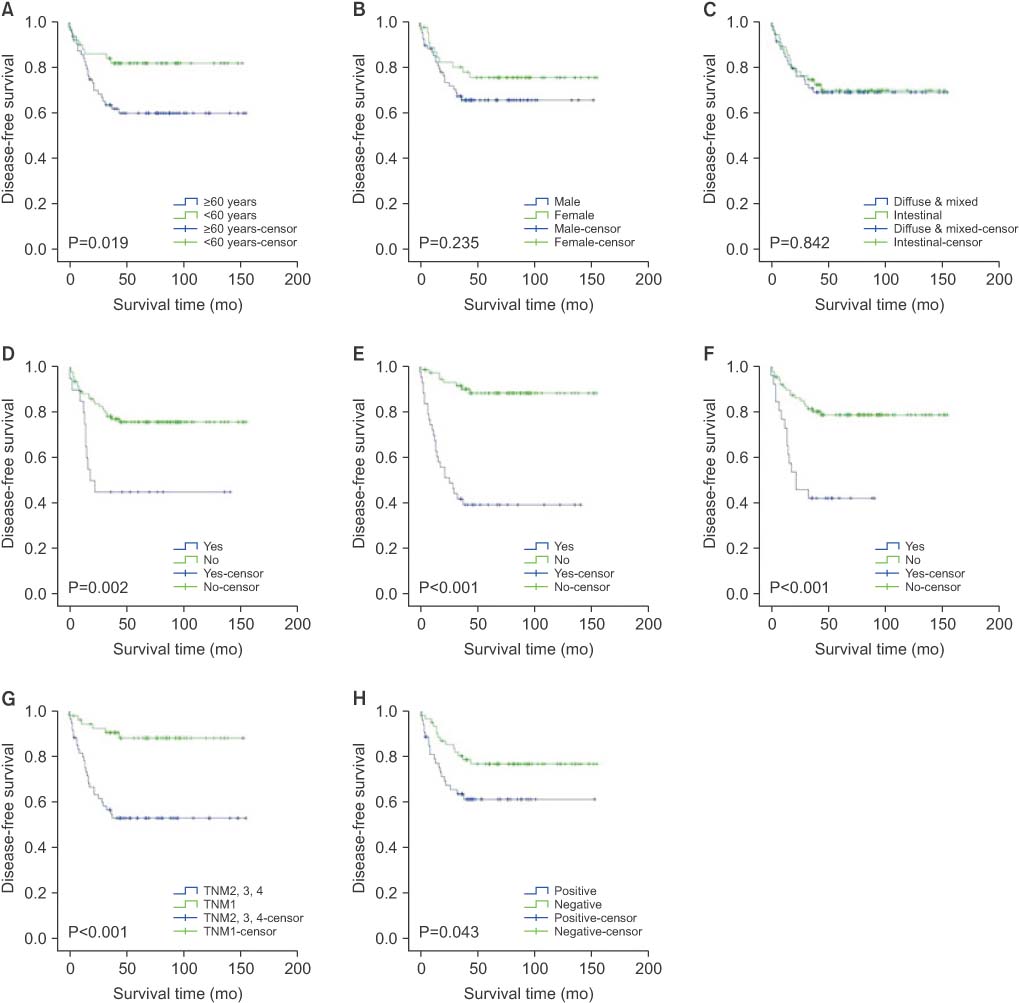
Among the final 143 subjects, 69 (48.3%) were CD44 positive. Older age, intestinal type gastric cancer, lymphatic invasion, and lymph node metastasis were significantly correlated with expression of CD44. In the multivariate analysis, older age was the only independent factor associated with CD44 expression (P=0.028). CD44 expression was correlated with overall survival, 5-year survival, and disease-free survival. In the multivariate analysis, older age, male gender, and lymphatic invasion were independent predictors of poor overall survival. Also, older age and lymphatic invasion were significant factors in 5-year survival, and lymphatic invasion was an independent factor of poor disease-free survival.
CONCLUSION
Older age (≥60 years) was independently associated with CD44 expression in gastric cancer patients. Also, CD44 expression was correlated with poor prognosis in gastric cancer patients.
MeSH Terms
Figure
Reference
-
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–E386.
Article2. Jung KW, Won YJ, Kong HJ, Oh CM, Cho H, Lee DH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015; 47:127–141.
Article3. Yasui W, Yokozaki H, Fujimoto J, Naka K, Kuniyasu H, Tahara E. Genetic and epigenetic alterations in multistep carcinogenesis of the stomach. J Gastroenterol. 2000; 35:Suppl 12. 111–115.4. Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, et al. Cancer stem cells: perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006; 66:9339–9344.5. Zhao Y, Feng F, Zhou YN. Stem cells in gastric cancer. World J Gastroenterol. 2015; 21:112–123.
Article6. Gomceli I, Demiriz B, Tez M. Gastric carcinogenesis. World J Gastroenterol. 2012; 18:5164–5170.7. Singh SR. Gastric cancer stem cells: a novel therapeutic target. Cancer Lett. 2013; 338:110–119.
Article8. Brungs D, Aghmesheh M, Vine KL, Becker TM, Carolan MG, Ranson M. Gastric cancer stem cells: evidence, potential markers, and clinical implications. J Gastroenterol. 2016; 51:313–326.
Article9. Nagano O, Saya H. Mechanism and biological significance of CD44 cleavage. Cancer Sci. 2004; 95:930–935.
Article10. Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, et al. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009; 27:1006–1020.
Article11. Ichikura T, Ogawa T, Kawabata T, Chochi K, Sugasawa H, Mochizuki H. Is adenocarcinoma of the gastric cardia a distinct entity independent of subcardial carcinoma? World J Surg. 2003; 27:334–338.
Article12. Kim MA, Lee HS, Yang HK, Kim WH. Clinicopathologic and protein expression differences between cardia carcinoma and noncardia carcinoma of the stomach. Cancer. 2005; 103:1439–1446.
Article13. Correa P. Human gastric carcinogenesis: a multistep and multifactorial process: first American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992; 52:6735–6740.14. Kimura K, Yoshida Y, Taniguchi Y, Ido K, Takemoto T. Chronological extension of atrophic gastritis and intestinal metaplasia in normal Japanese. Eur J Gastroenterol Hepatol. 1993; 5:85–91.15. Kwon KJ, Shim KN, Song EM, Choi JY, Kim SE, Jung HK, et al. Clinicopathological characteristics and prognosis of signet ring cell carcinoma of the stomach. Gastric Cancer. 2014; 17:43–53.
Article16. Hamilton SR, Aaltonen LA. Pathology and genetics of tumours of the digestive system. Lyon: International Agency for Research on Cancer;2000.17. Lopez-Carrillo L, Vega-Ramos B, Costa-Dias R, Rascon-Pacheco RA. Histological types of gastric cancer in Mexico. Int J Epidemiol. 1997; 26:1166–1171.
Article18. Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965; 64:31–49.19. Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010; 17:3077–3079.
Article20. van Diest PJ, van Dam Dam, Henzen-Logmans SC, Berns E, van der Burg ME, Green J, et al. A scoring system for immunohistochemical staining: consensus report of the task force for basic research of the EORTC-GCCG. European Organization for Research and Treatment of Cancer-Gynaecological Cancer Cooperative Group. J Clin Pathol. 1997; 50:801–804.
Article21. Dhingra S, Feng W, Brown RE, Zhou Z, Khoury T, Zhang R, et al. Clinicopathologic significance of putative stem cell markers, CD44 and nestin, in gastric adenocarcinoma. Int J Clin Exp Pathol. 2011; 4:733–741.22. Bu Z, Zheng Z, Zhang L, Li Z, Sun Y, Dong B, et al. LGR5 is a promising biomarker for patients with stage I and II gastric cancer. Chin J Cancer Res. 2013; 25:79–89.23. Khurana SS, Riehl TE, Moore BD, Fassan M, Rugge M, Romero-Gallo J, et al. The hyaluronic acid receptor CD44 coordinates normal and metaplastic gastric epithelial progenitor cell proliferation. J Biol Chem. 2013; 288:16085–16097.
Article24. Ghaffarzadehgan K, Jafarzadeh M, Raziee HR, Sima HR, Esmaili-Shandiz E, Hosseinnezhad H, et al. Expression of cell adhesion molecule CD44 in gastric adenocarcinoma and its prognostic importance. World J Gastroenterol. 2008; 14:6376–6381.
Article25. Lu L, Wu M, Sun L, Li W, Fu W, Zhang X, et al. Clinicopathological and prognostic significance of cancer stem cell markers CD44 and CD133 in patients with gastric cancer: A comprehensive meta-analysis with 4729 patients involved. Medicine (Baltimore). 2016; 95:e5163.26. Okayama H, Kumamoto K, Saitou K, Hayase S, Kofunato Y, Sato Y, et al. CD44v6, MMP-7 and nuclear Cdx2 are significant biomarkers for prediction of lymph node metastasis in primary gastric cancer. Oncol Rep. 2009; 22:745–755.
Article27. Kurozumi K, Nishida T, Nakao K, Nakahara M, Tsujimoto M. Expression of CD44 variant 6 and lymphatic invasion: importance to lymph node metastasis in gastric cancer. World J Surg. 1998; 22:853–857.
Article28. Wang W, Dong LP, Zhang N, Zhao CH. Role of cancer stem cell marker CD44 in gastric cancer: a meta-analysis. Int J Clin Exp Med. 2014; 7:5059–5066.29. Jung WY, Kang Y, Lee H, Mok YJ, Kim HK, Kim A, et al. Expression of moesin and CD44 is associated with poor prognosis in gastric adenocarcinoma. Histopathology. 2013; 63:474–481.
Article30. Wu Y, Li Z, Zhang C, Yu K, Teng Z, Zheng G, et al. CD44 family proteins in gastric cancer: a meta-analysis and narrative review. Int J Clin Exp Med. 2015; 8:3595–3606.31. Chen S, Hou JH, Feng XY, Zhang XS, Zhou ZW, Yun JP, et al. Clinicopathologic significance of putative stem cell marker, CD44 and CD133, in human gastric carcinoma. J Surg Oncol. 2013; 107:799–806.
Article32. Wakamatsu Y, Sakamoto N, Oo HZ, Naito Y, Uraoka N, Anami K, et al. Expression of cancer stem cell markers ALDH1, CD44 and CD133 in primary tumor and lymph node metastasis of gastric cancer. Pathol Int. 2012; 62:112–119.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CD44 and CD133 as Cancer Stem Cell Markers for Gastric Cancer
- Expression of CD44 Splicing Variants v4/5 and v6 in Gastric Adenocarcinoma and Its Relationship with Prognostic Factors
- Expression of CD44 in Preinvasive Disease and Invasive Cancer in Cervix
- The Role of CD44 in the Pathogenesis, Diagnosis, and Therapy of Gastric Cancer
- Expression of CD44 Standard, Variant 6 and Relationship to the Lymph Node Metastasis in Gastric Adenocarcinoma