J Vet Sci.
2018 Jul;19(4):519-527. 10.4142/jvs.2018.19.4.519.
An enhanced immunochromatographic strip test using colloidal gold nanoparticle-labeled dual-type N proteins for detection of antibodies to PRRS virus
- Affiliations
-
- 1Viral Disease Research Division, Animal and Plant Quarantine Agency, Gimcheon 39660, Korea. virusmania@korea.kr
- 2Research Institution, MEDIAN Diagnostics, Chuncheon 24399, Korea.
- KMID: 2417567
- DOI: http://doi.org/10.4142/jvs.2018.19.4.519
Abstract
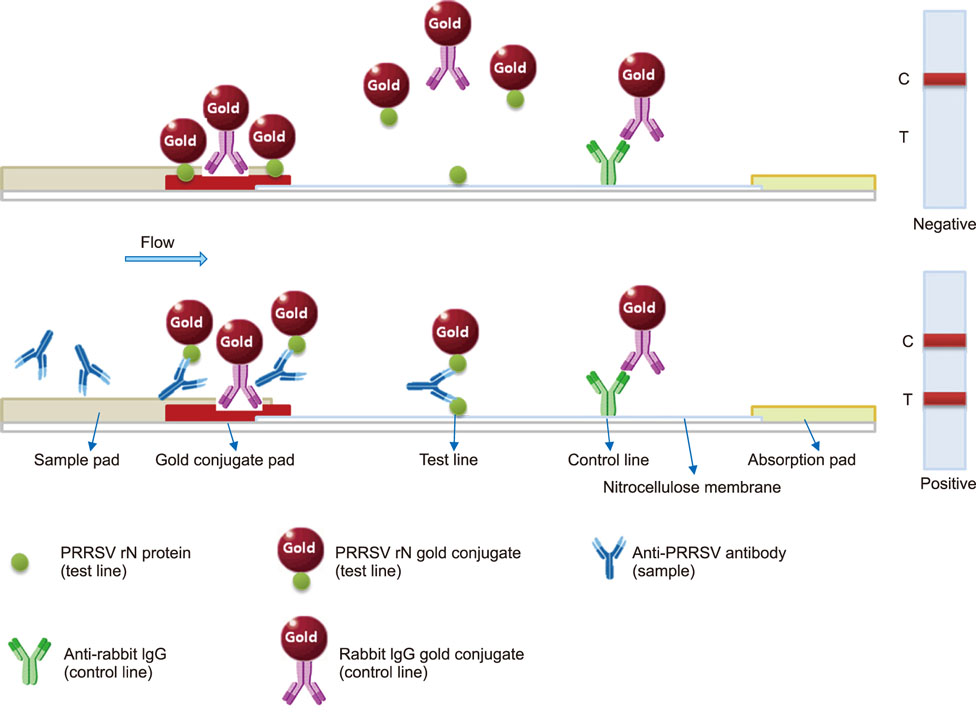
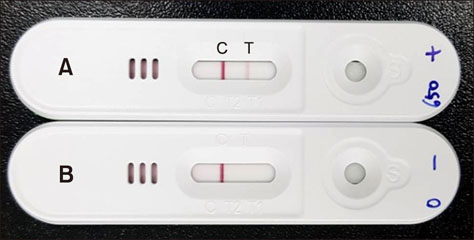
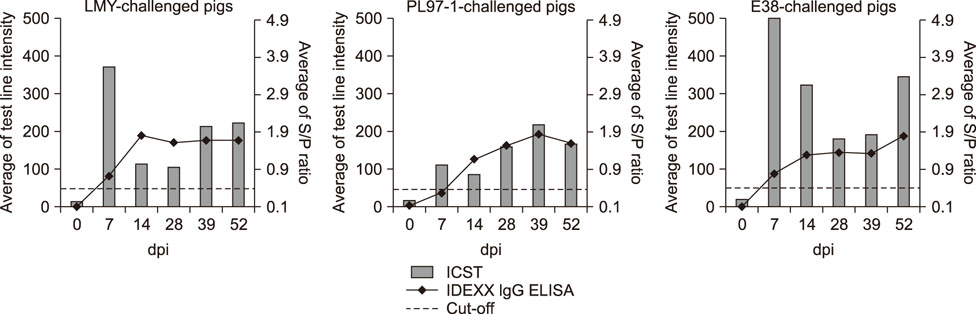
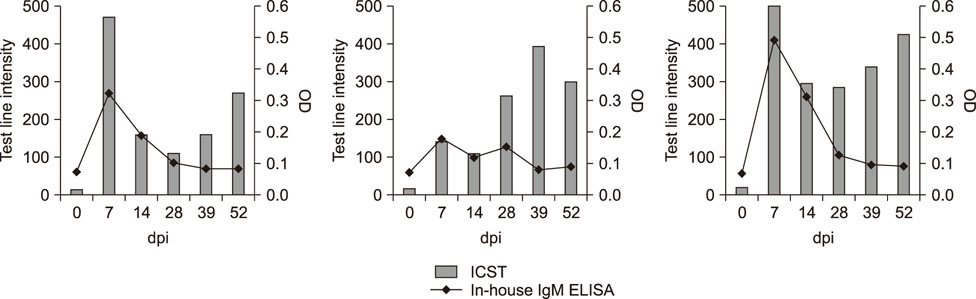
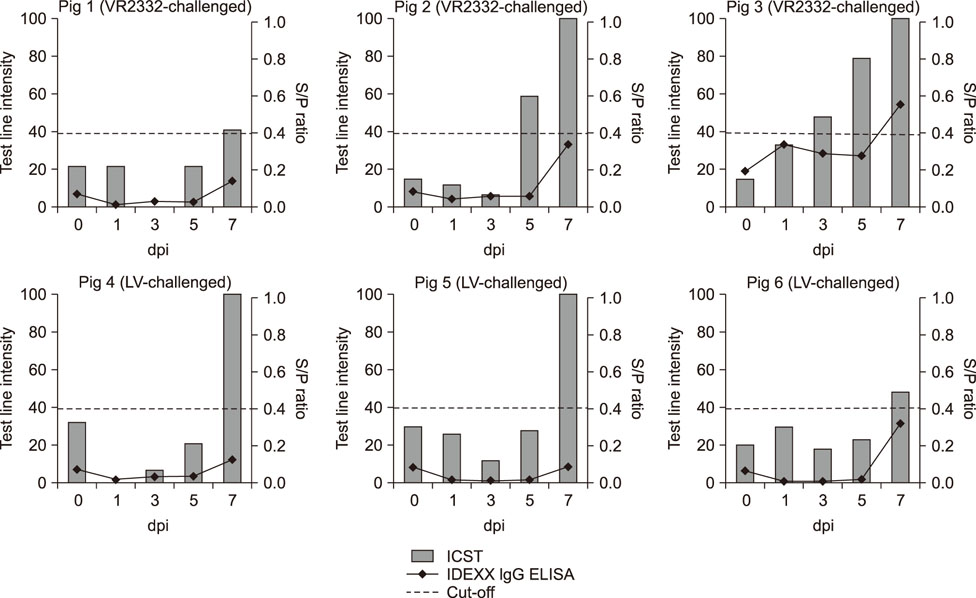
- Porcine reproductive and respiratory syndrome (PRRS) is recognized as one of the most important infectious diseases causing serious economic loss in the swine industry worldwide. Due to its increasing genetic diversity, a rapid and accurate diagnosis is critical for PRRS control. The immunochromatographic strip test (ICST) is a rapid and convenient type of immunoassay. In this study, an on-site immunochromatographic assay-based diagnostic method was developed for detection of PRRS virus (PRRSV)-specific antibodies. The method utilized colloidal gold nanoparticle-labeled dual-type nucleocapsid proteins encoded by open reading frame 7. We evaluated 991 field samples from pig farms and 66 serum samples from experimentally PRRSV-inoculated pigs. Based on true PRRSV-specific antibody-positive or -negative sera determined by immunofluorescence assay and IgM enzyme-linked immunosorbent assay (ELISA), the specificity and sensitivity of the ICST were 97.5% and 91.1%, respectively, similar to those of a commercial ELISA (IDEXX PRRS X3 Ab). More importantly, the ICST was completed within 15 min and could detect the PRRSV-specific antibody at an earlier stage of infection (3-7 days) than that of ELISA (7+ days). The results demonstrate that the developed ICST has great potential as an on-farm diagnostic method, providing excellent diagnostic performance in a quick and convenient manner.
Keyword
MeSH Terms
-
Agriculture
Antibodies*
Colloids*
Communicable Diseases
Diagnosis
Enzyme-Linked Immunosorbent Assay
Fluorescent Antibody Technique
Genetic Variation
Gold Colloid*
Immunoassay
Immunochromatography
Immunoglobulin M
Methods
Nucleocapsid Proteins
Open Reading Frames
Porcine Reproductive and Respiratory Syndrome*
Porcine respiratory and reproductive syndrome virus
Sensitivity and Specificity
Swine
Antibodies
Colloids
Gold Colloid
Immunoglobulin M
Nucleocapsid Proteins
Figure
Reference
-
1. Albina E. Epidemiology of porcine reproductive and respiratory syndrome (PRRS): an overview. Vet Microbiol. 1997; 55:309–316.
Article2. Beggs M, Novotny M, Sampedro S, Devore J, Gordon J, Osikowicz G. A self performing chromatographic immunoassay for the qualitative determination of human chorionic gonadotrophin (HCG) in urine and sera. Clin Chem. 1990; 36:1084–1085.3. Brown E, Lawson S, Welbon C, Gnanandarajah J, Li J, Murtaugh MP, Nelson EA, Molina RM, Zimmerman JJ, Rowland RR, Fang Y. Antibody response to porcine reproductive and respiratory syndrome virus (PRRSV) nonstructural proteins and implications for diagnostic detection and differentiation of PRRSV types I and II. Clin Vaccine Immunol. 2009; 16:628–635.
Article4. Carayannopoulos L, Capra ID. Immunoglobulins: structure and function. In : Paul WE, editor. Fundamental Immunology. 3rd ed. Raven Press: New York;1993. p. 283–314.5. Cha SH, Choi EJ, Park JH, Yoon SR, Song JY, Kwon JH, Song HJ, Yoon KJ. Molecular characterization of recent Korean porcine reproductive and respiratory syndrome (PRRS) viruses and comparison to other Asian PRRS viruses. Vet Microbiol. 2006; 117:248–257.
Article6. Choi EJ, Lee CH, Song JY, Song HJ, Park CK, Kim B, Shin YK. Genetic diversity of porcine reproductive and respiratory syndrome virus in Korea. J Vet Sci. 2013; 14:115–124.
Article7. Chu JQ, Hu XM, Kim MC, Park CS, Jun MH. Development and validation of a recombinant nucleocapsid protein-based ELISA for detection of the antibody to porcine reproductive and respiratory syndrome virus. J Microbiol. 2009; 47:582–588.
Article8. Cui S, Zhou S, Chen C, Qi T, Zhang C, Oh J. A simple and rapid immunochromatographic strip test for detecting antibody to porcine reproductive and respiratory syndrome virus. J Virol Methods. 2008; 152:38–42.
Article9. Dea S, Gagnon CA, Mardassi H, Pirzadeh B, Rogan D. Current knowledge on the structural proteins of porcine reproductive and respiratory syndrome (PRRS) virus: comparison of the North American and European isolates. Arch Virol. 2000; 145:659–688.
Article10. Díaz I, Darwich L, Pappaterra G, Pujols J, Mateu E. Immune responses of pigs after experimental infection with a European strain of porcine reproductive and respiratory syndrome virus. J Gen Virol. 2005; 86:1943–1951.
Article11. Holtkamp DJ, Kliebenstein JB, Neumann E, Zimmerman JJ, Rotto HF, Yoder TK, Wang C, Yeske PE, Mowrer CL, Haley CA. Assessment of the economic impact of porcine reproductive and respiratory syndrome virus on United States pork producers. J Swine Health Produc. 2013; 21:72–84.12. Joo HS, Park BK, Dee SA, Pijoan C. Indirect fluorescent IgM antibody response of pigs infected with porcine reproductive and respiratory syndrome syndrome virus. Vet Microbiol. 1997; 55:303–307.
Article13. Kameyama K, Sakoda Y, Tamai K, Igarashi H, Tajima M, Mochizuki T, Namba Y, Kida H. Development of an immunochromatographic test kit for rapid detection of bovine viral diarrhea virus antigen. J Virol Methods. 2006; 138:140–146.
Article14. Kim JY, Lee SY, Sur JH, Lyoo YS. Serological and genetic characterization of the European strain of the porcine reproductive and respiratory syndrome virus isolated in Korea. Korean J Vet Res. 2006; 46:363–370.15. Kim SH, Roh IS, Choi EJ, Lee C, Lee CH, Lee KH, Lee KK, Song YK, Lee OS, Park CK. A molecular analysis of European porcine reproductive and respiratory syndrome virus isolated in South Korea. Vet Microbiol. 2010; 143:394–400.
Article16. Kittawornrat A, Engle M, Panyasing Y, Olsen C, Schwartz K, Rice A, Lizano S, Wang C, Zimmerman J. Kinetics of the porcine reproductive and respiratory syndrome virus (PRRSV) humoral immune response in swine serum and oral fluids collected from individual boars. BMC Vet Res. 2013; 9:61.
Article17. Labarque GG, Nauwynck HJ, Van Reeth K, Pensaert MB. Effect of cellular changes and onset of humoral immunity on the replication of porcine reproductive and respiratory syndrome virus in the lungs of pigs. J Gen Virol. 2000; 81:1327–1334.
Article18. Lee JA, Lee NH, Lee JB, Park SY, Song CS, Choi IS, Lee SW. Genetic diversity of the Korean field strains of porcine reproductive and respiratory syndrome virus. Infect Genet Evol. 2016; 40:288–294.
Article19. Li Y, Hou L, Ye J, Liu X, Dan H, Jin M, Chen H, Cao S. Development of a convenient immunochromatographic strip for the diagnosis of infection with Japanese encephalitis virus in swine. J Virol Methods. 2010; 168:51–56.
Article20. Loemba HD, Mounir S, Mardassi H, Archambault D, Dea S. Kinetics of humoral immune response to the major structural proteins of the porcine reproductive and respiratory syndrome virus. Arch Virol. 1996; 141:751–761.
Article21. Magar R, Larochelle R, Robinson Y, Dubuc C. Immunohistochemical detection of porcine reproductive and respiratory syndrome virus using colloidal gold. Can J Vet Res. 1993; 57:300–304.22. Mateu E, Diaz I. The challenge of PRRS immunology. Vet J. 2008; 177:345–351.
Article23. Mengeling WL, Lager KM, Vorwald AC. Clinical effects of porcine reproductive and respiratory syndrome virus on pigs during the early postnatal interval. Am J Vet Res. 1998; 59:52–55.24. Nodelijk G, Wensvoort G, Kroese B, van Leengoed L, Colijn E, Verheijden J. Comparison of a commercial ELISA and an immunoperoxidase monolayer assay to detect antibodies directed against porcine respiratory and reproductive syndrome virus. Vet Microbiol. 1996; 49:285–295.
Article25. Okinaga T, Yamagishi T, Yoshii M, Suzuki T, Miyazaki A, Takagi M, Tsunemitsu H. Evaluation of unexpected positive results from a commercial ELISA for antibodies to PRRSV. Vet Rec. 2009; 164:455–459.
Article26. Peng D, Hu S, Hua Y, Xiao Y, Li Z, Wang X, Bi D. Comparison of a new gold-immunochromatographic assay for the detection of antibodies against avian influenza virus with hemagglutination inhibition and agar gel immunodiffusion assays. Vet Immunol Immunopathol. 2007; 117:17–25.
Article27. Peng F, Wang Z, Zhang S, Wu R, Hu S, Li Z, Wang X, Bi D. Development of an immunochromatographic strip for rapid detection of H9 subtype avian influenza viruses. Clin Vaccine Immunol. 2008; 15:569–574.
Article28. Sattler T, Wodak E, Revilla-Fernández S, Schmoll F. Comparison of different commercial ELISAs for detection of antibodies against porcine respiratory and reproductive syndrome virus in serum. BMC Vet Res. 2014; 10:300.
Article29. Sithigorngul W, Rukpratanporn S, Sittidilokratna N, Pecharaburanin N, Longyant S, Chaivisuthangkura P, Sithigorngul P. A convenient immunochromatographic test strip for rapid diagnosis of yellow head virus infection in shrimp. J Virol Methods. 2007; 140:193–199.
Article30. Tanaka R, Yuhi T, Nagatani N, Endo T, Kerman K, Takamura Y, Tamiya E. A novel enhancement assay for immunochromatographic test strips using gold nanoparticles. Anal Bioanal Chem. 2006; 385:1414–1420.
Article31. Vézina SA, Loemba H, Fournier M, Dea S, Archambault D. Antibody production and blastogenic response in pigs experimentally infected with porcine reproductive and respiratory syndrome virus. Can J Vet Res. 1996; 60:94–99.32. Yoon KJ, Zimmerman JJ, Swenson SL, McGinley MJ, Eernisse KA, Brevik A, Rhinehart LL, Frey ML, Hill HT, Platt KB. Characterization of the humoral immune response to porcine reproductive and respiratory syndrome (PRRS) virus infection. J Vet Diagn Invest. 1995; 7:305–312.
Article33. Zhou SH, Cui SJ, Chen CM, Zhang FC, Li J, Zhou S, Oh JS. Development and validation of an immunogold chromatographic test for on-farm detection of PRRSV. J Virol Methods. 2009; 160:178–184.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development of an immunochromatographic strip for detection of antibodies against porcine reproductive and respiratory syndrome virus
- Evaluation of a Rapid Detection Influenza Virus A Antigens Kit Using Paired Serum Antibody Test
- A rapid and quantitative fluorescent microsphere immunochromatographic strip test for detection of antibodies to porcine reproductive and respiratory syndrome virus
- Evaluation of a Simple Immunochromatographic Assay as Qualitative Screening Test for Anti-PGL-I Antibodies in Serodiagnosis of Leprosy (I)
- Effects of Colloidal Gold 198Au on Synovial Membrane of Rabbits