Yonsei Med J.
2013 Mar;54(2):476-479. 10.3349/ymj.2013.54.2.476.
Evaluation of a Rapid Detection Influenza Virus A Antigens Kit Using Paired Serum Antibody Test
- Affiliations
-
- 1Department of Respiratory Medicine, The Military General Hospital of Beijing PLA (The Chinese People's Liberation Army, PLA), Beijing, China. hw-chen@hotmail.com
- 2Department of Respiratory Medicine, Beijing Pinggu Hospital, Beijing, China.
- 3Beijing Centers for Diseases Control and Prevention (CDC) & Centers for Preventive Medical Research, Beijing, China.
- KMID: 1503913
- DOI: http://doi.org/10.3349/ymj.2013.54.2.476
Abstract
- PURPOSE
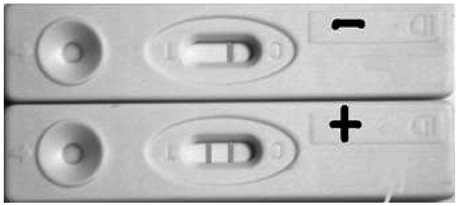
To evaluate the feasibility for gold immunochromatographic assay (GICA) in rapid detection of influenza virus A infection.
MATERIALS AND METHODS
Seventy-three patients were enrolled. All patients contributed nasopharyngeal secretions and paired serum samples. Nasopharyngeal secretions was used for colloidal gold immunochromatographic rapid assay for influenza A virus immediately after the collection of specimen. Paired serum samples were used for the hemagglutination inhibition assay at the Centers for Disease Control and Prevention influenza network laboratory in Beijing.
RESULTS
Compare GICA test to hemagglutination inhibition (HI) assay, the Kappa value was 0.402 and the p value in the paired chi2 test was higher than 0.05. Therefore, the difference was not statistically significant. The sensitivity of GICA was 50.0% and the specificity was 90.2%, and the negative predictive value was 90.2%.
CONCLUSION
The sensitivity for Influenza A antigen detection by using GICA is relatively low, the specificity is relatively satisfactory.
MeSH Terms
Figure
Reference
-
1. Lamb RA, Krug RM. Orthomyxoviridae: the viruses and their replication. Fields Virology. 2001. Philadelphia (PA): Lippincott Williams & Wilkins;1487–1531.2. Hindiyeh M, Levy V, Azar R, Varsano N, Regev L, Shalev Y, et al. Evaluation of a multiplex real-time reverse transcriptase PCR assay for detection and differentiation of influenza viruses A and B during the 2001-2002 influenza season in Israel. J Clin Microbiol. 2005. 43:589–595.
Article3. Kilbourne ED. Influenza pandemics of the 20th century. Emerg Infect Dis. 2006. 12:9–14.
Article4. Guan Y, Vijaykrishna D, Bahl J, Zhu H, Wang J, Smith GJ. The emergence of pandemic influenza viruses. Protein Cell. 2010. 1:9–13.
Article5. Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009. 459:1122–1125.
Article6. Trifonov V, Khiabanian H, Greenbaum B, Rabadan R. The origin of the recent swine influenza A(H1N1) virus infecting humans. Euro Surveill. 2009. 14:pii: 19193.
Article7. Dwyer DE, Smith DW, Catton MG, Barr IG. Laboratory diagnosis of human seasonal and pandemic influenza virus infection. Med J Aust. 2006. 185:10 Suppl. S48–S53.
Article8. Bonner AB, Monroe KW, Talley LI, Klasner AE, Kimberlin DW. Impact of the rapid diagnosis of influenza on physician decision-making and patient management in the pediatric emergency department: results of a randomized, prospective, controlled trial. Pediatrics. 2003. 112:363–367.
Article9. World Health Organization. WHO recommendations on the use of rapid testing for influenza diagnosis. Available at: http://www.who.int/influenza/resources/documents/rapid_testing/en/.10. Petric M, Comanor L, Petti CA. Role of the laboratory in diagnosis of influenza during seasonal epidemics and potential pandemics. J Infect Dis. 2006. 194:Suppl 2. S98–S110.
Article11. Heeschen C, Goldmann BU, Moeller RH, Hamm CW. Analytical performance and clinical application of a new rapid bedside assay for the detection of serum cardiac troponin I. Clin Chem. 1998. 44:1925–1930.
Article12. Watanabe T, Ohkubo Y, Matsuoka H, Kimura H, Sakai Y, Ohkaru Y, et al. Development of a simple whole blood panel test for detection of human heart-type fatty acid-binding protein. Clin Biochem. 2001. 34:257–263.
Article13. Li X, Chen H, Wei J, Lv N, You L. The evaluation of colloidal gold immunochromatographic assay (GICA) for rapid diagnosis of influenza A disease. Clin Chem Lab Med. 2011. 49:1533–1537.
Article14. Shafir SC, O'Keefe KA, Shoaf KI. Evaluation of the seroprevalence of influenza A(H1N1) 2009 on a university campus: a cross-sectional study. BMC Public Health. 2011. 11:922.
Article15. Ministry of Health of the People's Republic of China. Program of diagnosis and treatment of influenza A H1N1(2010). Int J Respir. 2011. 31. (In Chinese).
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of a Rapid Antigen Test for Detection of Influenza Virus
- Sensitivities of seasonal and pandemic rapid antigen tests differentiated by the level of immunofluorescence for the detection of pandemic influenza A/H1N1 2009 virus
- Comparison of Rapid Antigen Test and Real-Time Reverse Transcription PCR for the Detection of Influenza B Virus
- Clinical Analysis of Influenza in Children and Rapid Antigen Detection Test on First Half of the Year 2004 in Busan
- Evaluation of the Efficacies of Rapid Antigen Test, Multiplex PCR, and Real-time PCR for the Detection of a Novel Influenza A (H1N1) Virus