Obstet Gynecol Sci.
2018 May;61(3):337-343. 10.5468/ogs.2018.61.3.337.
Optimal cutoff level of serum squamous cell carcinoma antigen to detect recurrent cervical squamous cell carcinoma during post-treatment surveillance
- Affiliations
-
- 1Department of Obstetrics and Gynecology, School of Medicine, Catholic University of Daegu, Daegu, Korea. duchess7@naver.com
- KMID: 2416121
- DOI: http://doi.org/10.5468/ogs.2018.61.3.337
Abstract
OBJECTIVE
The objective of this study was to determine the optimal cutoff level of serum squamous cell carcinoma antigen (SCC-Ag) to detect recurrent cervical squamous cell carcinoma during post-treatment surveillance.
METHODS
Between January 2000 and July 2014, a total of 158 women with cervical squamous cell carcinoma were treated with radiotherapy with or without concurrent chemotherapy at our department. A total of 1,550 serum SCC-Ag tests performed during post-treatment surveillance of the 158 patients were included in this retrospective study.
RESULTS
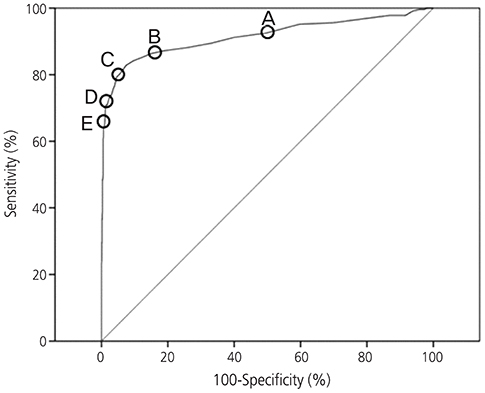
During post-treatment surveillance, 53 patients were diagnosed as having recurrent cervical cancer based on biopsy or a radiological test showing progression of a lesion. Receiver operating characteristic (ROC) curve for serum SCC-Ag to diagnose recurrent cervical squamous cell carcinoma showed that the area under the ROC curve was 0.914 (95% confidence interval, 0.887-0.942; P < 0.001). The best cutoff value for serum SCC-Ag to obtain the highest Youden's index was ≥2 ng/mL (sensitivity, 80.2%; specificity, 94.6%).
CONCLUSION
Serum SCC-Ag test was helpful in detecting recurrent cervical squamous cell carcinoma during post-treatment surveillance, and the optimal cutoff value was ≥2 ng/mL. The researchers recommend active imaging studies, when serum SCC-Ag level ≥2 ng/mL during post-treatment surveillance.
MeSH Terms
Figure
Reference
-
1. Practice bulletin no. 157: cervical cancer screening and prevention. Obstet Gynecol. 2016; 127:e1–e20.2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.
Article3. Salani R, Backes FJ, Fung MF, Holschneider CH, Parker LP, Bristow RE, et al. Posttreatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncologists recommendations. Am J Obstet Gynecol. 2011; 204:466–478.
Article4. Zanagnolo V, Ming L, Gadducci A, Maggino T, Sartori E, Zola P, et al. Surveillance procedures for patients with cervical carcinoma: a review of the literature. Int J Gynecol Cancer. 2009; 19:194–201.
Article5. Duk JM, van Voorst Vader PC, ten Hoor KA, Hollema H, Doeglas HM, de Bruijn HW. Elevated levels of squamous cell carcinoma antigen in patients with a benign disease of the skin. Cancer. 1989; 64:1652–1656.
Article6. Gadducci A, Tana R, Cosio S, Genazzani AR. The serum assay of tumour markers in the prognostic evaluation, treatment monitoring and follow-up of patients with cervical cancer: a review of the literature. Crit Rev Oncol Hematol. 2008; 66:10–20.
Article7. Jao MS, Chang TC, Chang HP, Wu TI, Chao A, Lai CH. Long-term follow up of cervical cancer patients with unexplained squamous cell carcinoma antigen elevation after post-therapy surveillance using positron emission tomography. J Obstet Gynaecol Res. 2010; 36:1003–1008.
Article8. Chan YM, Ng TY, Ngan HY, Wong LC. Monitoring of serum squamous cell carcinoma antigen levels in invasive cervical cancer: is it cost-effective? Gynecol Oncol. 2002; 84:7–11.
Article9. Zola P, Macchi C, Cibula D, Colombo N, Kimmig R, Maggino T, et al. Follow-up in gynecological malignancies: a state of art. Int J Gynecol Cancer. 2015; 25:1151–1164.
Article10. Micke O, Prott FJ, Schafer U, Tangerding S, Potter R, Willich N. The impact of squamous cell carcinoma (SCC) antigen in the follow-up after radiotherapy in patients with cervical cancer. Anticancer Res. 2000; 20:5113–5115.11. Meier W, Eiermann W, Stieber P, Schneider A, Fateh-Moghadam A, Hepp H. Experiences with SCC antigen, a new tumor marker for cervical carcinoma. Eur J Cancer Clin Oncol. 1989; 25:1555–1559.
Article12. Senekjian EK, Young JM, Weiser PA, Spencer CE, Magic SE, Herbst AL. An evaluation of squamous cell carcinoma antigen in patients with cervical squamous cell carcinoma. Am J Obstet Gynecol. 1987; 157:433–439.
Article13. Forni F, Ferrandina G, Deodato F, Macchia G, Morganti AG, Smaniotto D, et al. Squamous cell carcinoma antigen in follow-up of cervical cancer treated with radiotherapy: evaluation of cost-effectiveness. Int J Radiat Oncol Biol Phys. 2007; 69:1145–1149.
Article14. Ogino I, Nakayama H, Kitamura T, Okamoto N, Inoue T. The curative role of radiotherapy in patients with isolated para-aortic node recurrence from cervical cancer and value of squamous cell carcinoma antigen for early detection. Int J Gynecol Cancer. 2005; 15:630–638.
Article15. Reesink-Peters N, van der Velden J, Ten Hoor KA, Boezen HM, de Vries EG, Schilthuis MS, et al. Preoperative serum squamous cell carcinoma antigen levels in clinical decision making for patients with early-stage cervical cancer. J Clin Oncol. 2005; 23:1455–1462.
Article16. Shimura K, Mabuchi S, Yokoi T, Sasano T, Sawada K, Hamasaki T, et al. Utility of serum squamous cell carcinoma antigen levels at the time of recurrent cervical cancer diagnosis in determining the optimal treatment choice. J Gynecol Oncol. 2013; 24:321–329.
Article17. Strauss HG, Laban C, Lautenschlager C, Buchmann J, Schneider I, Koelbl H. SCC antigen in the serum as an independent prognostic factor in operable squamous cell carcinoma of the cervix. Eur J Cancer. 2002; 38:1987–1991.
Article18. Ryu HK, Baek JS, Kang WD, Kim SM. The prognostic value of squamous cell carcinoma antigen for predicting tumor recurrence in cervical squamous cell carcinoma patients. Obstet Gynecol Sci. 2015; 58:368–376.
Article19. Elit L, Fyles AW, Devries MC, Oliver TK, Fung-Kee-Fung M. Gynecology Cancer Disease Site G. Follow-up for women after treatment for cervical cancer: a systematic review. Gynecol Oncol. 2009; 114:528–535.20. Morice P, Deyrolle C, Rey A, Atallah D, Pautier P, Camatte S, et al. Value of routine follow-up procedures for patients with stage I/II cervical cancer treated with combined surgery-radiation therapy. Ann Oncol. 2004; 15:218–223.
Article21. Coleman RL, Keeney ED, Freedman RS, Burke TW, Eifel PJ, Rutledge FN. Radical hysterectomy for recurrent carcinoma of the uterine cervix after radiotherapy. Gynecol Oncol. 1994; 55:29–35.
Article22. Berek JS, Howe C, Lagasse LD, Hacker NF. Pelvic exenteration for recurrent gynecologic malignancy: survival and morbidity analysis of the 45-year experience at UCLA. Gynecol Oncol. 2005; 99:153–159.
Article23. Singh AK, Grigsby PW, Rader JS, Mutch DG, Powell MA. Cervix carcinoma, concurrent chemoradiotherapy, and salvage of isolated paraaortic lymph node recurrence. Int J Radiat Oncol Biol Phys. 2005; 61:450–455.
Article24. Niibe Y, Kenjo M, Kazumoto T, Michimoto K, Takayama M, Yamauchi C, et al. Multi-institutional study of radiation therapy for isolated para-aortic lymph node recurrence in uterine cervical carcinoma: 84 subjects of a population of more than 5,000. Int J Radiat Oncol Biol Phys. 2006; 66:1366–1369.
Article25. Kim JS, Kim JS, Kim SY, Kim K, Cho MJ. Hyperfractionated radiotherapy with concurrent chemotherapy for para-aortic lymph node recurrence in carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2003; 55:1247–1253.
Article26. Anraku M, Yokoi K, Nakagawa K, Fujisawa T, Nakajima J, Akiyama H, et al. Pulmonary metastases from uterine malignancies: results of surgical resection in 133 patients. J Thorac Cardiovasc Surg. 2004; 127:1107–1112.
Article27. Salvatici M, Achilarre MT, Sandri MT, Boveri S, Vanna Z, Landoni F. Squamous cell carcinoma antigen (SCC-Ag) during follow-up of cervical cancer patients: Role in the early diagnosis of recurrence. Gynecol Oncol. 2016; 142:115–119.
Article28. Kainz C, Sliutz G, Mustafa G, Bieglmayr C, Koelbl H, Reinthaller A, et al. Cytokeratin subunit 19 measured by CYFRA 21-1 assay in follow-up of cervical cancer. Gynecol Oncol. 1995; 56:402–405.
Article29. Kotowicz B, Fuksiewicz M, Jonska-Gmyrek J, Bidzinski M, Kowalska M. The assessment of the prognostic value of tumor markers and cytokines as SCCAg, CYFRA 21.1, IL-6, VEGF and sTNF receptors in patients with squamous cell cervical cancer, particularly with early stage of the disease. Tumour Biol. 2016; 37:1271–1278.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clninical Utility of Serum Squamous Cell Carcinoma Antigen and Urine Polyamines in Cervical Carcinoma
- Clinical value of routine serum squamous cell carcinoma antigen in follow-up of patients with locally advanced cervical cancer treated with radiation or chemoradiation
- A Case of Ovarian Squamous Cell Carcinoma in a Patient with Microinvasive Squamous Cell Carcinoma of Cervix
- Pseudoangiosarcomatous Squamous Cell Carcinoma of the Face
- The Clinical Significance of Squamous Cell Carcinoma Antigen as a Predictor of Nodal Metastasis in Early Stage Cervical Carcinoma