Investig Magn Reson Imaging.
2018 Jun;22(2):79-85. 10.13104/imri.2018.22.2.79.
Pseudoglandular Formation in Hepatocellular Carcinoma Determines Apparent Diffusion Coefficient in Diffusion-Weighted MRI
- Affiliations
-
- 1Department of Radiology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. yjsrad97@yuhs.ac
- KMID: 2415885
- DOI: http://doi.org/10.13104/imri.2018.22.2.79
Abstract
- PURPOSE
To determine the impact of pseudoglandular formation on apparent diffusion coefficient (ADC) values of hepatocellular carcinoma (HCC) in diffusion-weighted imaging (DWI), and to validate the results using histopathological grades.
MATERIALS AND METHODS
We assessed 182 HCCs surgically resected from 169 consecutive patients. Each type of tumor pseudoglandular formation was categorized into "non-,""mixed-," or "pure-," based on official histopathology reports. The ADC for each tumor was independently measured, using the largest region of interest on the ADC map. Data were assessed using the analysis of variance test, with Bonferroni correction for post hoc analysis to stratify the relationship of ADCs with pseudoglandular formation, followed by subgroup analysis according to the histopathological tumor grades.
RESULTS
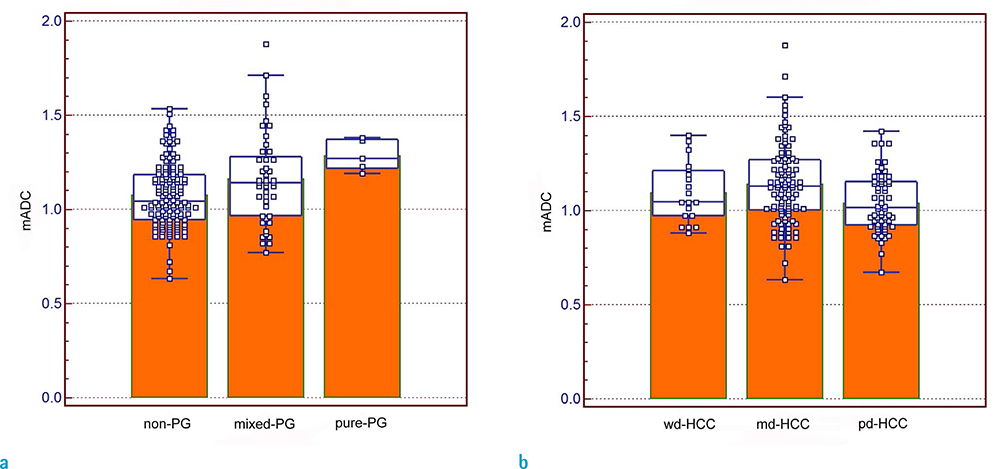
The mean ADC was significantly higher in pure pseudoglandular lesions (n = 5, 1.29 ± 0.08 × 10−3 mm2/s) than in non-pseudoglandular lesions (n = 132, 1.08 ± 0.17 × 10−3 mm2/s; P = 0.003) or mixed-pseudoglandular lesions (n = 45, 1.16 ± 0.24 × 10−3 mm2/s; P = 0.034). The ADC values and pseudoglandular formation were significantly correlated in moderately differentiated HCCs (n = 103; r = 0.307, P = 0.007), while well- (n = 19) and poorly-differentiated HCCs (n = 60) did not show significant correlation (r = 0.105 and 0.068, respectively; P = 0.600 and 0.685, respectively).
CONCLUSION
The degree of pseudoglandular formation could be one of the determinants of ADC in DWI of HCCs-especially moderately differentiated HCCs-while its influence does not appear to be significant in well- or poorly differentiated HCCs.
Keyword
MeSH Terms
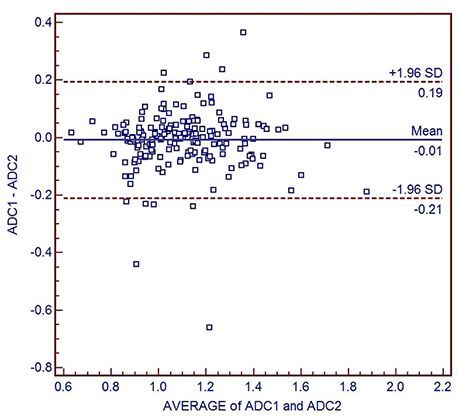
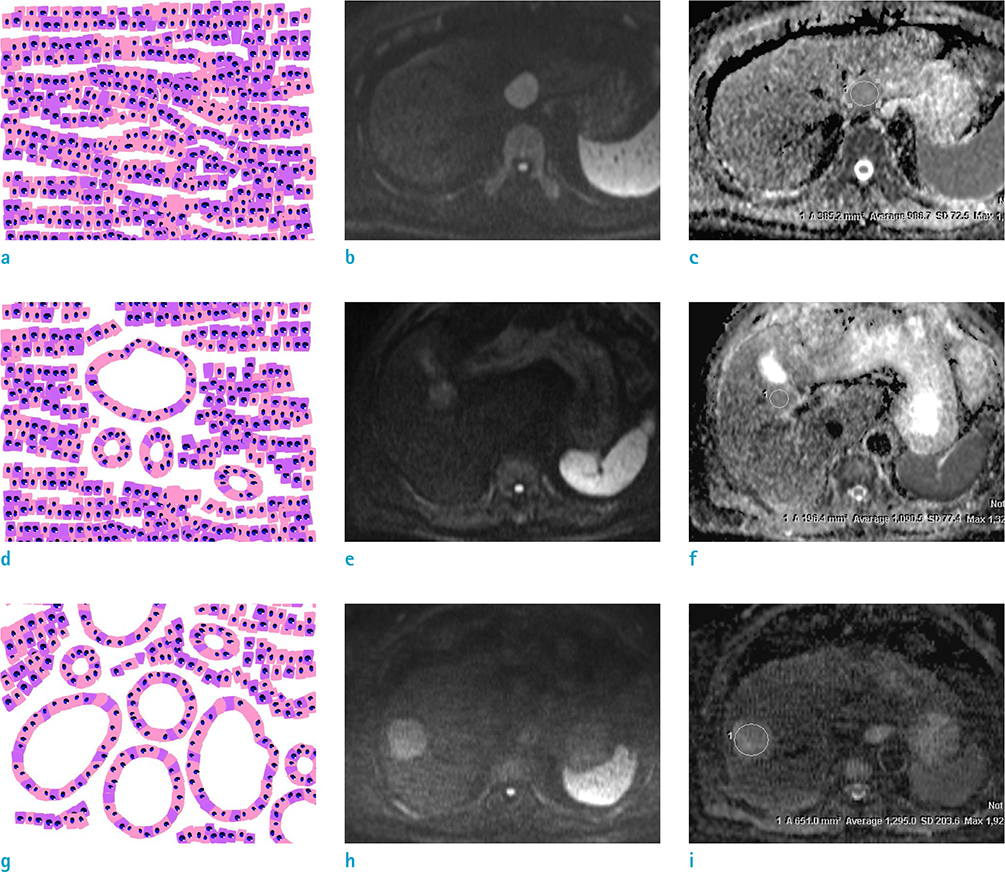
Figure
Reference
-
1. Rampone B, Schiavone B, Martino A, Viviano C, Confuorto G. Current management strategy of hepatocellular carcinoma. World J Gastroenterol. 2009; 15:3210–3216.
Article2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.
Article3. Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005; 53:1432–1440.
Article4. Kele PG, van der Jagt EJ. Diffusion weighted imaging in the liver. World J Gastroenterol. 2010; 16:1567–1576.
Article5. Parikh T, Drew SJ, Lee VS, et al. Focal liver lesion detection and characterization with diffusion-weighted MR imaging: comparison with standard breath-hold T2-weighted imaging. Radiology. 2008; 246:812–822.
Article6. Lowenthal D, Zeile M, Lim WY, et al. Detection and characterisation of focal liver lesions in colorectal carcinoma patients: comparison of diffusion-weighted and Gd-EOB-DTPA enhanced MR imaging. Eur Radiol. 2011; 21:832–840.
Article7. Lee MH, Kim SH, Park MJ, Park CK, Rhim H. Gadoxetic acid-enhanced hepatobiliary phase MRI and high-b-value diffusion-weighted imaging to distinguish well-differentiated hepatocellular carcinomas from benign nodules in patients with chronic liver disease. AJR Am J Roentgenol. 2011; 197:W868–W875.
Article8. Inchingolo R, De Gaetano AM, Curione D, et al. Role of diffusion-weighted imaging, apparent diffusion coefficient and correlation with hepatobiliary phase findings in the differentiation of hepatocellular carcinoma from dysplastic nodules in cirrhotic liver. Eur Radiol. 2015; 25:1087–1096.
Article9. Muhi A, Ichikawa T, Motosugi U, et al. High-b-value diffusion-weighted MR imaging of hepatocellular lesions: estimation of grade of malignancy of hepatocellular carcinoma. J Magn Reson Imaging. 2009; 30:1005–1011.
Article10. Heo SH, Jeong YY, Shin SS, et al. Apparent diffusion coefficient value of diffusion-weighted imaging for hepatocellular carcinoma: correlation with the histologic differentiation and the expression of vascular endothelial growth factor. Korean J Radiol. 2010; 11:295–303.
Article11. Nishie A, Tajima T, Asayama Y, et al. Diagnostic performance of apparent diffusion coefficient for predicting histological grade of hepatocellular carcinoma. Eur J Radiol. 2011; 80:e29–e33.
Article12. Chang WC, Chen RC, Chou CT, et al. Histological grade of hepatocellular carcinoma correlates with arterial enhancement on gadoxetic acid-enhanced and diffusion-weighted MR images. Abdom Imaging. 2014; 39:1202–1212.
Article13. Chen J, Wu M, Liu R, Li S, Gao R, Song B. Preoperative evaluation of the histological grade of hepatocellular carcinoma with diffusion-weighted imaging: a meta-analysis. PLoS One. 2015; 10:e0117661.
Article14. Guo W, Zhao S, Yang Y, Shao G. Histological grade of hepatocellular carcinoma predicted by quantitative diffusion-weighted imaging. Int J Clin Exp Med. 2015; 8:4164–4169.15. Tang Y, Wang H, Ma L, et al. Diffusion-weighted imaging of hepatocellular carcinomas: a retrospective analysis of correlation between apparent diffusion coefficients and histological grade. Abdom Radiol (NY). 2016; 41:1539–1545.
Article16. Li X, Zhang K, Shi Y, Wang F, Meng X. Correlations between the minimum and mean apparent diffusion coefficient values of hepatocellular carcinoma and tumor grade. J Magn Reson Imaging. 2016; 44:1442–1447.
Article17. Noworolski SM, Vigneron DB, Chen AP, Kurhanewicz J. Dynamic contrast-enhanced MRI and MR diffusion imaging to distinguish between glandular and stromal prostatic tissues. Magn Reson Imaging. 2008; 26:1071–1080.
Article18. Wang Y, Chen ZE, Nikolaidis P, et al. Diffusion-weighted magnetic resonance imaging of pancreatic adenocarcinomas: association with histopathology and tumor grade. J Magn Reson Imaging. 2011; 33:136–142.
Article19. Yu JS, Kim JH, Chung JJ, Kim KW. Added value of diffusion-weighted imaging in the MRI assessment of perilesional tumor recurrence after chemoembolization of hepatocellular carcinomas. J Magn Reson Imaging. 2009; 30:153–160.
Article20. Theise ND, Park YN, Curado MP, et al. Hepatocellular carcinoma. In : Bosman FT, Carneiro F, Hruban RH, editors. WHO classification of tumours of the digestive system. 4th ed. Lyon: International Agency for Research on Cancer;2010. p. 205–216.21. Woo S, Lee JM, Yoon JH, Joo I, Han JK, Choi BI. Intravoxel incoherent motion diffusion-weighted MR imaging of hepatocellular carcinoma: correlation with enhancement degree and histologic grade. Radiology. 2014; 270:758–767.
Article22. Granata V, Fusco R, Catalano O, et al. Intravoxel incoherent motion (IVIM) in diffusion-weighted imaging (DWI) for hepatocellular carcinoma: correlation with histologic grade. Oncotarget. 2016; 7:79357–79364.
Article23. Shan Q, Chen J, Zhang T, et al. Evaluating histologic differentiation of hepatitis B virus-related hepatocellular carcinoma using intravoxel incoherent motion and AFP levels alone and in combination. Abdom Radiol (NY). 2017; 42:2079–2208.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reversal of a Large Ischemic Lesion with Low Apparent Diffusion Coefficient Value by Rapid Spontaneous Recanalization
- Dysarthria with Hypoglycemia and Reversible Focal Hyperintensity Lesion on Diffusion?Weighted MRI
- Diffusion-weighted Imaging and Apparent Diffusion Coefficient Maps for the Evaluation of Pyogenic Ventriculitis
- Diffusion-Weighted MRI in Intrahepatic Bile Duct Adenoma Arising from the Cirrhotic Liver
- Serial Magnetic Resonance Images of a Right Middle Cerebral Artery Infarction : Persistent Hyperintensity on Diffusion-Weighted MRI Over 8 Months