J Periodontal Implant Sci.
2018 Jun;48(3):182-192. 10.5051/jpis.2018.48.3.182.
Tissue integration of zirconia and titanium implants with and without buccal dehiscence defects
- Affiliations
-
- 1Clinic of Fixed and Removable Prosthodontics and Dental Material Science, University of Zurich, Zurich, Switzerland.
- 2Department of Periodontology, Research Institute for Periodontal Regeneration, Yonsei University College of Dentistry, Seoul, Korea. drjew@yuhs.ac
- KMID: 2414931
- DOI: http://doi.org/10.5051/jpis.2018.48.3.182
Abstract
- PURPOSE
The purpose of the present study was to validate an experimental model for assessing tissue integration of titanium and zirconia implants with and without buccal dehiscence defects.
METHODS
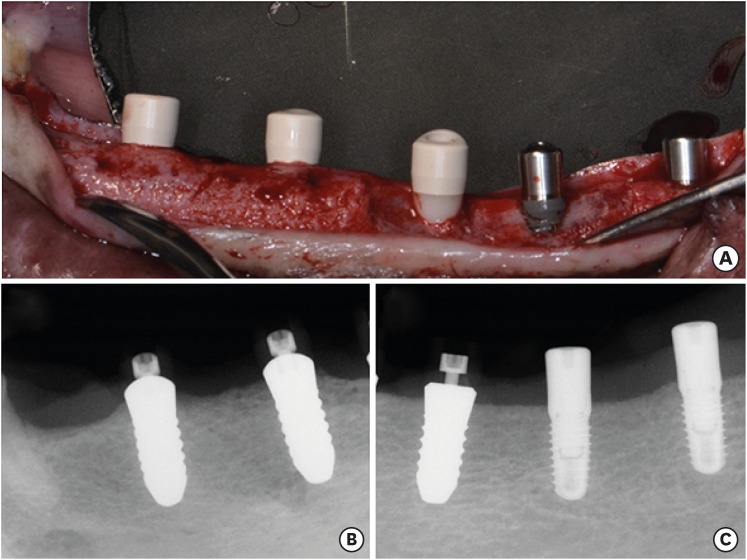
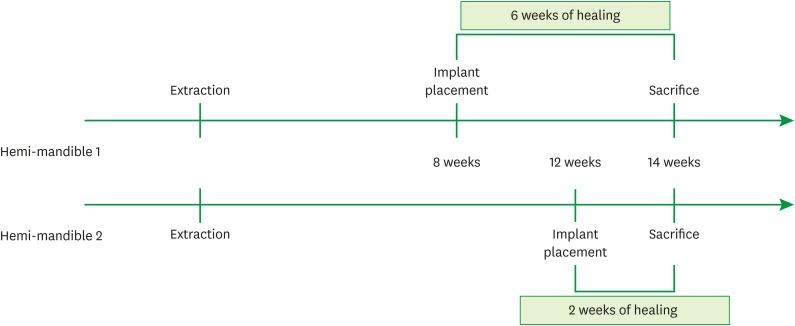
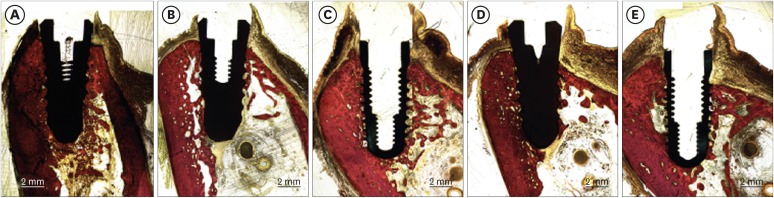
In 3 dogs, 5 implants were randomly placed on both sides of the mandibles: 1) Z1: a zirconia implant (modified surface) within the bony housing, 2) Z2: a zirconia implant (standard surface) within the bony housing, 3) T: a titanium implant within the bony housing, 4) Z1_D: a Z1 implant placed with a buccal bone dehiscence defect (3 mm), and 5) T_D: a titanium implant placed with a buccal bone dehiscence defect (3 mm). The healing times were 2 weeks (one side of the mandible) and 6 weeks (the opposite side).
RESULTS
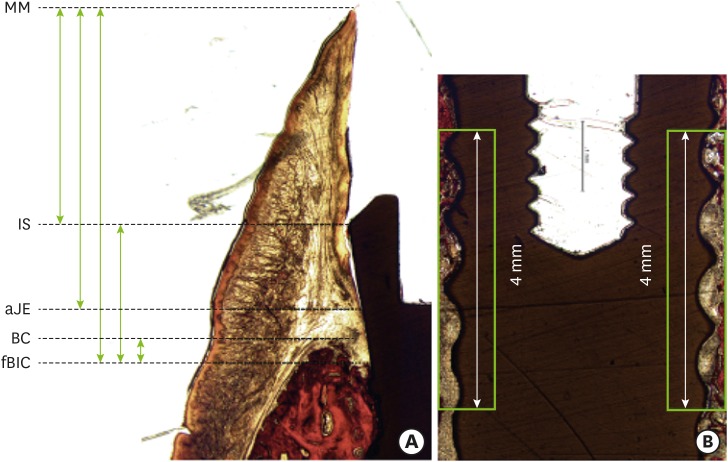
The dimensions of the peri-implant soft tissue varied depending on the implant and the healing time. The level of the mucosal margin was located more apically at 6 weeks than at 2 weeks in all groups, except group T. The presence of a buccal dehiscence defect did not result in a decrease in the overall soft tissue dimensions between 2 and 6 weeks (4.80±1.31 and 4.3 mm in group Z1_D, and 4.47±1.06 and 4.5±1.37 mm in group T_D, respectively). The bone-to-implant contact (BIC) values were highest in group Z1 at both time points (34.15%±21.23% at 2 weeks, 84.08%±1.33% at 6 weeks). The buccal dehiscence defects in groups Z1_D and T_D showed no further bone loss at 6 weeks compared to 2 weeks.
CONCLUSIONS
The modified surface of Z1 demonstrated higher BIC values than the surface of Z2. There were minimal differences in the mucosal margin between 2 and 6 weeks in the presence of a dehiscence defect. The present model can serve as a useful tool for studying peri-implant dehiscence defects at the hard and soft tissue levels.
MeSH Terms
Figure
Reference
-
1. Buser D, Sennerby L, De Bruyn H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontol 2000. 2017; 73:7–21. PMID: 28000280.
Article2. Bosshardt DD, Chappuis V, Buser D. Osseointegration of titanium, titanium alloy and zirconia dental implants: current knowledge and open questions. Periodontol 2000. 2017; 73:22–40. PMID: 28000277.
Article3. Jung RE, Zembic A, Pjetursson BE, Zwahlen M, Thoma DS. Systematic review of the survival rate and the incidence of biological, technical, and aesthetic complications of single crowns on implants reported in longitudinal studies with a mean follow-up of 5 years. Clin Oral Implants Res. 2012; 23(Suppl 6):2–21. PMID: 23062124.4. Müller K, Valentine-Thon E. Hypersensitivity to titanium: clinical and laboratory evidence. Neuroendocrinol Lett. 2006; 27(Suppl 1):31–35.5. Toumelin-Chemla F, Rouelle F, Burdairon G. Corrosive properties of fluoride-containing odontologic gels against titanium. J Dent. 1996; 24:109–115. PMID: 8636481.
Article6. van Brakel R, Noordmans HJ, Frenken J, de Roode R, de Wit GC, Cune MS. The effect of zirconia and titanium implant abutments on light reflection of the supporting soft tissues. Clin Oral Implants Res. 2011; 22:1172–1178. PMID: 21251080.
Article7. Cionca N, Hashim D, Mombelli A. Zirconia dental implants: where are we now, and where are we heading? Periodontol 2000. 2017; 73:241–258. PMID: 28000266.
Article8. Liñares A, Grize L, Muñoz F, Pippenger BE, Dard M, Domken O, et al. Histological assessment of hard and soft tissues surrounding a novel ceramic implant: a pilot study in the minipig. J Clin Periodontol. 2016; 43:538–546. PMID: 26969899.
Article9. Thoma DS, Benic GI, Muñoz F, Kohal R, Sanz Martin I, Cantalapiedra AG, et al. Histological analysis of loaded zirconia and titanium dental implants: an experimental study in the dog mandible. J Clin Periodontol. 2015; 42:967–975. PMID: 26362505.
Article10. Thoma DS, Benic GI, Muñoz F, Kohal R, Sanz Martin I, Cantalapiedra AG, et al. Marginal bone-level alterations of loaded zirconia and titanium dental implants: an experimental study in the dog mandible. Clin Oral Implants Res. 2016; 27:412–420. PMID: 25864523.
Article11. Kohal RJ, Weng D, Bächle M, Strub JR. Loaded custom-made zirconia and titanium implants show similar osseointegration: an animal experiment. J Periodontol. 2004; 75:1262–1268. PMID: 15515343.
Article12. Sennerby L, Dasmah A, Larsson B, Iverhed M. Bone tissue responses to surface-modified zirconia implants: A histomorphometric and removal torque study in the rabbit. Clin Implant Dent Relat Res. 2005; 7(Suppl 1):S13–S20. PMID: 16137083.
Article13. Tetè S, Mastrangelo F, Bianchi A, Zizzari V, Scarano A. Collagen fiber orientation around machined titanium and zirconia dental implant necks: an animal study. Int J Oral Maxillofac Implants. 2009; 24:52–58. PMID: 19344025.14. Welander M, Abrahamsson I, Berglundh T. The mucosal barrier at implant abutments of different materials. Clin Oral Implants Res. 2008; 19:635–641. PMID: 18492075.
Article15. Jung RE, Herzog M, Wolleb K, Ramel CF, Thoma DS, Hämmerle CH. A randomized controlled clinical trial comparing small buccal dehiscence defects around dental implants treated with guided bone regeneration or left for spontaneous healing. Clin Oral Implants Res. 2017; 28:348–354. PMID: 26923088.
Article16. Benic GI, Mokti M, Chen CJ, Weber HP, Hämmerle CH, Gallucci GO. Dimensions of buccal bone and mucosa at immediately placed implants after 7 years: a clinical and cone beam computed tomography study. Clin Oral Implants Res. 2012; 23:560–566. PMID: 22093013.17. Kuchler U, Chappuis V, Gruber R, Lang NP, Salvi GE. Immediate implant placement with simultaneous guided bone regeneration in the esthetic zone: 10-year clinical and radiographic outcomes. Clin Oral Implants Res. 2016; 27:253–257. PMID: 25906924.
Article18. Donath K, Breuner G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Säge-Schliff (sawing and grinding) technique. J Oral Pathol. 1982; 11:318–326. PMID: 6809919.19. Abrahamsson I, Berglundh T, Moon IS, Lindhe J. Peri-implant tissues at submerged and non-submerged titanium implants. J Clin Periodontol. 1999; 26:600–607. PMID: 10487311.
Article20. Abrahamsson I, Berglundh T, Wennström J, Lindhe J. The peri-implant hard and soft tissues at different implant systems. A comparative study in the dog. Clin Oral Implants Res. 1996; 7:212–219. PMID: 9151585.
Article21. Cochran DL, Mau LP, Higginbottom FL, Wilson TG, Bosshardt DD, Schoolfield J, et al. Soft and hard tissue histologic dimensions around dental implants in the canine restored with smaller-diameter abutments: a paradigm shift in peri-implant biology. Int J Oral Maxillofac Implants. 2013; 28:494–502. PMID: 23527352.
Article22. Sculean A, Gruber R, Bosshardt DD. Soft tissue wound healing around teeth and dental implants. J Clin Periodontol. 2014; 41(Suppl 15):S6–22. PMID: 24641001.
Article23. Berglundh T, Abrahamsson I, Welander M, Lang NP, Lindhe J. Morphogenesis of the peri-implant mucosa: an experimental study in dogs. Clin Oral Implants Res. 2007; 18:1–8. PMID: 17224016.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Influence of kilovoltage- peak and the metal artifact reduction tool in cone-beam computed tomography on the detection of bone defects around titanium-zirconia and zirconia implants
- Comparison of marginal and internal fit of zirconia abutments with titanium abutments in internal hexagonal implants
- Zirconia Abutment Fracture in the Anterior Region: Case Series
- On the osseointegration of zirconia and titanium implants installed at defect site filled with xenograft material
- Effect of coloring treatment of translucent zirconia on the masking ability of metal abutment