Transl Clin Pharmacol.
2018 Jun;26(2):86-92. 10.12793/tcp.2018.26.2.86.
The first step to the powers for clinical trials: a survey on the current and future Clinical Trial Management System
- Affiliations
-
- 1Center for Clinical Pharmacology and Biomedical Research Institute, Chonbuk National University Hospital, Jeonju 54907, Republic of Korea. mgkim@jbnu.ac.kr
- 2Department of Pharmacology, School of Medicine, Chonbuk National University, Jeonju 54907, Republic of Korea.
- KMID: 2413832
- DOI: http://doi.org/10.12793/tcp.2018.26.2.86
Abstract
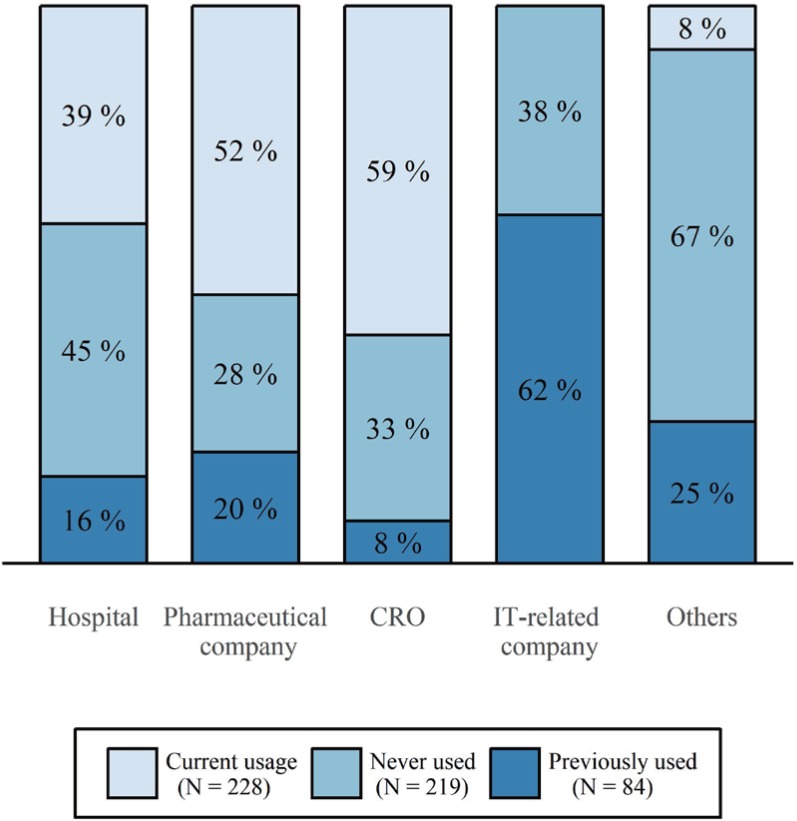
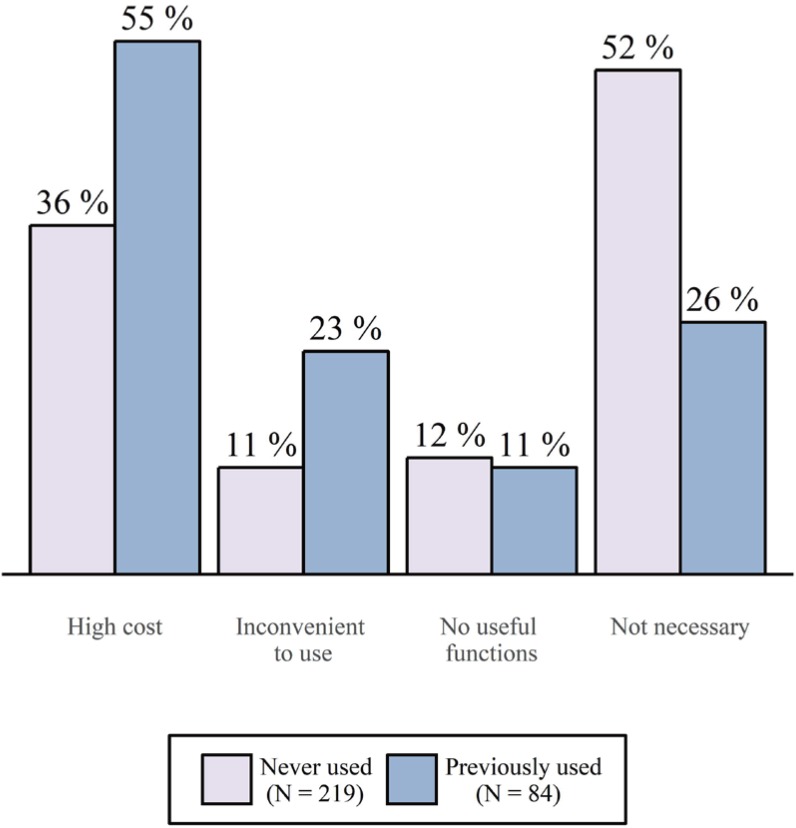
- A clinical trial management system (CTMS) is a comprehensive program that supports an efficient clinical trial. To improve the environment of clinical trials and to be competitive in the global clinical trials market, an advanced and integrated CTMS is necessary. However, there is little information about the status of CTMSs in Korea. To understand the utilization of current CTMSs and requirements for a future CTMS, we conducted a survey on the subjects related to clinical trials. The survey was conducted from July 27 to August 16, 2017. The total number of respondents was 596, and 531 of these responses were used. Almost half of the respondents were from hospitals (46%). The proportion of respondents who are currently using a CTMS was the highest for contract research organizations at 59%, whereas the proportion used by investigators was 39%. The main reason for not using a CTMS was that it is unnecessary and expensive, but it showed a difference between workplaces. Many respondents frequently used CTMSs to check the clinical trial schedule and progress status, which was needed regardless of workplace. While two-thirds of users tended to be satisfied with their current CTMS, there were many users who felt their CTMS was inconvenient. The most requested function for a future CTMS was one that could be used to manage the project schedule and subject enrollment status. Additionally, a systematic linkage to electronic medical records, including prescription and laboratory test results, and a function to confirm the participation history of subjects in other hospitals were requested.
Keyword
MeSH Terms
Figure
Reference
-
1. Integrated addendum to ICH E6 (R1): Guideline for Good Clinical Practice E6 (R2). Accessed 2 February 2018. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R2__Step_4_2016_1109.pdf/.2. Status of clinical trials overseas. Accessed 25 May 2018. https://www.koreaclinicaltrials.org/kr/contents/datainfo_data_01_tab03/view.do/.3. START WITH KOREA. Accessed 21 May 2018. https://www.konect.or.kr/upload/downfile/Start_with_Korea_2017.pdf/.4. Background and Necessity. Accessed 23 May 2018. http://eng.kcgi.or.kr/sub1/index2.htm/.5. Announcement of Clinical Trial Strengthening Plan of global competitiveness. Accessed 6 February 2018. http://www.mohw.go.kr/react/al/sal0301vw.jsp?PAR_MENU_ID=04&MENU_ID=0403&CONT_SEQ=325129&page=1/.6. THE POWER OF CTMS. Accessed 20 February 2018. http://www.oracle.com/us/industries/life-sciences/oracle-life-sciences-ctms-1625650.pdf.7. Park YR, Yoon YJ, Koo H, Yoo S, Choi CM, Beck SH, et al. Utilization of a Clinical Trial Management System for the Whole Clinical Trial Process as an Integrated Database: System Development. J Med Internet Res. 2018; 20:e103. DOI: 10.2196/jmir.9312. PMID: 29691212.
Article8. Wang BR, Choi IY. Current State and Applications of the Electronic Clinical Trial Process in Korea. J Korea Contents Assoc. 2013; 13:281–289.
Article9. Effoe VS, Katula JA, Kirk JK, Pedley CF, Bollhalter LY, Brown WM, et al. The use of electronic medical records for recruitment in clinical trials: findings from the Lifestyle Intervention for Treatment of Diabetes trial. Trials. 2016; 17:496. PMID: 27733193.
Article10. Weng C, Li Y, Berhe S, Boland MR, Gao J, Hruby GW, et al. An Integrated Model for Patient Care and Clinical Trials (IMPACT) to support clinical research visit scheduling workflow for future learning health systems. J Biomed Inform. 2013; 46:642–652. PMID: 23684593.
Article11. Campion TR Jr, Blau VL, Brown SW, Izcovich D, Cole CL. Implementing a Clinical Research Management System: One Institution's Successful Approach Following Previous Failures. AMIA Jt Summits Transl Sci Proc. 2014; 2014:12–17. PMID: 25954570.12. Choi YJ. A Study on the Regulation and Legal Issues of Medical Information Cloud Computing : Comparison with Proposal for General Data Protection Regulation of 2012 in the European Union. Eur Const. 2017; 25:105–138.13. KCGI starts to develop the smart clinical trial core platform. Accessed 16 May 2018. http://www.kcgi.or.kr/sub4/data/read.htm?bn=data&fmlid=225&pkid=15&board_no=225&thisPage=1&startTextId=&buffer=/.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Trends of clinical trials from 2014 to 2016 in South Korea
- Survey of Risk-Based Quality Management Status andEstablishment of Operational Model in Clinical Trials
- Clinical trials and ethics
- A Study on Current Status of Clinical Trial Pharmacy in Domestic Clinical Trial Institution
- Analysis of the distribution of trial sites in South Korea using social network analysis