J Vet Sci.
2016 Dec;17(4):489-496. 10.4142/jvs.2016.17.4.489.
Two clinical isolates of Mycoplasma hyosynoviae showed differing pattern of lameness and pathogen detection in experimentally challenged pigs
- Affiliations
-
- 1Department of Veterinary Diagnostic and Production Animal Medicine, College of Veterinary Medicine, Iowa State University, Ames, IA 50011, USA. jgneto84@huskers.unl.edu
- 2Food Science and Technology, University of Nebraska Lincoln, Lincoln, NE 68588, USA.
- 3Wisconsin National Primate Research Center, University of Wisconsin, Madison, WI 53703, USA.
- 4Merck Animal Health, De Soto, KS 66018, USA.
- 5Zoetis Inc., Global Biologics Research, Kalamazoo, MI 49007, USA.
- KMID: 2412604
- DOI: http://doi.org/10.4142/jvs.2016.17.4.489
Abstract
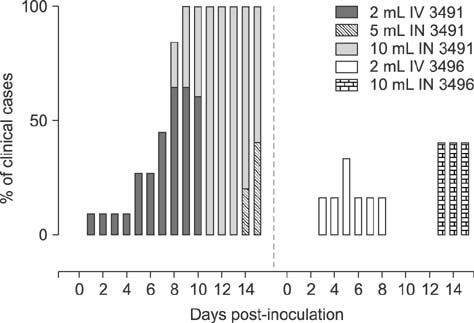
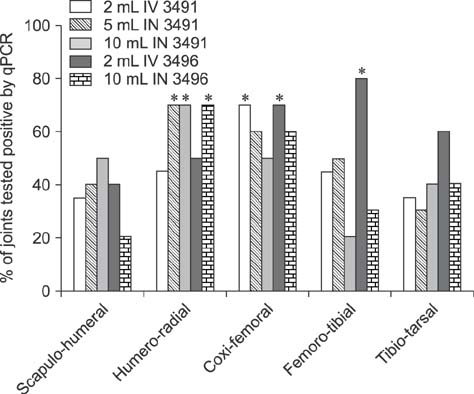
- Mycoplasma (M.) hyosynoviae is known to colonize and cause disease in growing-finishing pigs. In this study, two clinical isolates of M. hyosynoviae were compared by inoculating cesarean-derived colostrum-deprived and specific-pathogen-free growing pigs. After intranasal or intravenous inoculation, the proportion and distribution pattern of clinical cases was compared in addition to the severity of lameness. Tonsils were found to be the primary site of colonization, while bacteremia was rarely detected prior to the observation of clinical signs. Regardless of the clinical isolate, route of inoculation, or volume of inocula, histopathological alterations and tissue invasion were detected in multiple joints, indicating an apparent lack of specific joint tropism. Acute disease was primarily observed 7 to 10 days post-inoculation. The variability in the severity of synovial microscopic lesions and pathogen detection in joint cavities suggests that the duration of joint infection may influence the diagnostic accuracy. In summary, these findings demonstrate that diagnosis of M. hyosynoviae-associated arthritis can be influenced by the clinical isolate, and provides a study platform to investigate the colonization and virulence potential of field isolates. This approach can be particularly relevant to auxiliate in surveillance and testing of therapeutic and/or vaccine candidates.
Keyword
MeSH Terms
-
Acute Disease
Animals
Arthritis, Infectious/epidemiology/microbiology/*veterinary
Colostrum
Lameness, Animal/*epidemiology/microbiology
Mycoplasma Infections/epidemiology/microbiology/*veterinary
Mycoplasma hyosynoviae/genetics/*physiology
Specific Pathogen-Free Organisms
Swine
Swine Diseases/*epidemiology/microbiology
Figure
Reference
-
1. Bumgardner EA, Kittichotirat W, Bumgarner RE, Lawrence PK. Comparative genomic analysis of seven Mycoplasma hyosynoviae strains. Microbiologyopen. 2015; 4:343–359.
Article2. Friis NF, Ahrens P, Larsen H. Mycoplasma hyosynoviae isolation from the upper respiratory tract and tonsil of pigs. Acta Vet Scand. 1991; 32:425–429.
Article3. Gomes Neto JC, Bower L, Erickson BZ, Wang C, Raymond M, Strait EL. Quantitative real-time polymerase chain reaction for detecting of Mycoplasma hyosynoviae and Mycoplasma hyorhinis in pen-based oral, tonsillar and nasal fluids. J Vet Sci. 2015; 16:195–201.
Article4. Gomes Neto JC, Gauger PC, Strait EL, Boyes N, Madson DM, Schwartz KJ. Mycoplasma-associated arthritis: critical points for diagnosis. J Swine Health Prod. 2012; 20:82–86.5. Gomes Neto JC, Strait EL, Raymond M, Ramirez A, Minion FC. Antibody responses of swine following infection with Mycoplasma hyopneumoniae, M. hyorhinis, M. hyosynoviae and M. flocculare. Vet Microbiol. 2014; 174:163–171.6. Hagedorn-Olsen T, Basse A, Jensen TK, Nielsen NC. Gross and histopathological findings in synovial membranes of pigs with experimentally induced Mycoplasma hyosynoviae arthritis. APMIS. 1999; 107:201–210.
Article7. Hagedorn-Olsen T, Nielsen NC, Friis NF. Induction of arthritis with Mycoplasma hyosynoviae in pigs: clinical response and re-isolation of the organism from body fluids and organs. Zentralbl Veterinarmed A. 1999; 46:317–325.
Article8. Hagedorn-Olsen T, Nielsen NC, Friis NF, Nielsen J. Progression of Mycoplasma hyosynoviae infection in three pigs herds. Development of tonsillar carrier state, arthritis, and antibodies in serum and synovial fluid in pigs from birth to slaughter. Zentralbl Veterinarmed A. 1999; 46:555–564.9. Holt RD. Population dynamics in two-patch environments: some anomalous consequences of an optimal habitat distribution. Theor Popul Biol. 1985; 28:181–208.
Article10. Kobisch M, Friis NF. Swine mycoplasmoses. Rev Sci Tech. 1996; 15:1569–1605.
Article11. Kokotovic B, Friis NF, Nielsen EO, Ahrens P. Genomic diversity among Danish field strains of Mycoplasma hyosynoviae assessed by amplified fragment length polymorphism analysis. Vet Microbiol. 2002; 85:221–231.
Article12. Lauritsen KT, Hagedorn-Olsen T, Friis NF, Lind P, Jungersen G. Absence of strictly age-related resistance to Mycoplasma hyosynoviae infection in 6-week-old pigs. Vet Microbiol. 2008; 130:385–390.
Article13. Nielsen EO, Nielsen NC, Friis NF. Mycoplasma hyosynoviae in grower-finisher pigs. J Vet Med A Physiol Pathol Clin Med. 2001; 48:475–486.14. Pulliam HR. Sources, sinks, and population regulation. Am Nat. 1988; 132:652–661.
Article15. Ross RF. Predisposing factors in Mycoplasma hyosynoviae arthritis of swine. J Infect Dis. 1973; 127:Suppl. S84–S86.16. Ross RF, Duncan JR. Mycoplasma hyosynoviae arthritis of swine. J Am Vet Med Assoc. 1970; 157:1515–1518.17. Ross RF, Spear ML. Role of the sow as a reservoir of infection for Mycoplasma hyosynoviae. Am J Vet Res. 1973; 34:373–378.18. Ross RF, Switzer WP, Duncan JR. Experimental production of Mycoplasma hyosynoviae arthritis in swine. Am J Vet Res. 1971; 32:1743–1749.19. Stakenborg T, Vicca J, Butaye P, Imberechts H, Peeters J, de Kruif A, Haesebrouck F, Maes D. A multiplex PCR to identify porcine mycoplasmas present in broth cultures. Vet Res Commun. 2006; 30:239–247.
Article20. Stemke GW, Robertson JA. Comparison of two methods for enumeration of mycoplasmas. J Clin Microbiol. 1982; 16:959–961.
Article21. Strait EL, Madsen ML, Minion FC, Christopher-Hennings J, Dammen M, Jones KR, Thacker EL. Real-Time PCR assays to address genetic diversity among strains of Mycoplasma hyopneumoniae. J Clin Microbiol. 2008; 46:2491–2498.
Article22. Thacker EL, Minion FC. Mycoplasmosis. In : Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, editors. Diseases of Swine. 10th ed. Ames: Wiley-Blackwell;2012. p. 779–797.23. Wills RW, Zimmerman JJ, Yoon KJ, Swenson SL, McGinley MJ, Hill HT, Platt KB, Christopher-Hennings J, Nelson EA. Porcine reproductive and respiratory syndrome virus: a persistent infection. Vet Microbiol. 1997; 55:231–240.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Quantitative real-time polymerase chain reaction for detecting Mycoplasma hyosynoviae and Mycoplasma hyorhinis in pen-based oral, tonsillar, and nasal fluids
- Efficacy of bivalent vaccines of porcine circovirus type 2 and Mycoplasma hyopneumoniae in specific pathogen-free pigs challenged with porcine circovirus type 2d
- Molecular subtyping and antimicrobial susceptibility of Streptococcus dysgalactiae subspecies equisimilis isolates from clinically diseased pigs
- A sensitive and specific polymerase chain reaction to detect Mycoplasma hyopnemoniae using Mycoplasma protein P97 gene
- Diagnosis of Mycoplasma hyorhinis infection in pigs by PCR amplification of 16S-23S rRNA internal transcribed spacer region