J Vet Sci.
2015 Jun;16(2):195-201. 10.4142/jvs.2015.16.2.195.
Quantitative real-time polymerase chain reaction for detecting Mycoplasma hyosynoviae and Mycoplasma hyorhinis in pen-based oral, tonsillar, and nasal fluids
- Affiliations
-
- 1Department of Veterinary Diagnostic and Production Animal Medicine, College of Veterinary Medicine, Iowa State University, Ames, IA 5011, USA. jgneto84@huskers.unl.edu
- 2352 Food Science Complex, University of Nebraska, Lincoln, NE 68583, USA.
- 3Wisconsin National Primate Research Center, University of Wisconsin, Madison, WI 53715, USA.
- 4Merck Animal Health, DeSoto, KS 66018, USA.
- KMID: 2164529
- DOI: http://doi.org/10.4142/jvs.2015.16.2.195
Abstract
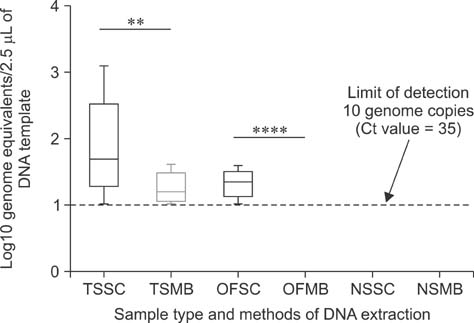
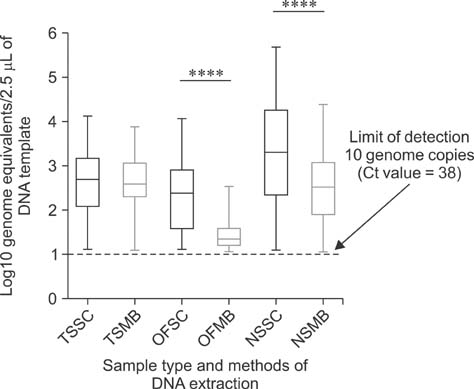
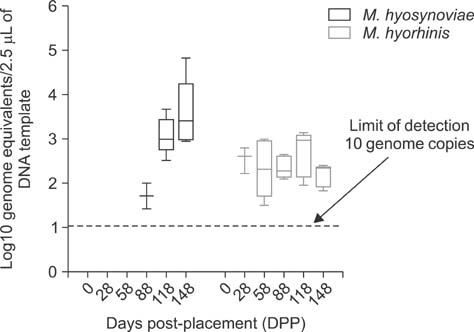
- Mycoplasma (M.) hyorhinis and M. hyosynoviae are pathogens known to cause disease in pigs post-weaning. Due to their fastidious nature, there is increased need for culture-independent diagnostic platforms to detect these microorganisms. Therefore, this study was performed to develop and optimize quantitative real-time PCR (qPCR) assays to rapidly detect M. hyorhinis and M. hyosynoviae in pen-based oral fluids as well as nasal and tonsillar fluids as proxies for samples used in swine herd surveillance. Two methods of genomic DNA extraction, automated versus manual, were used to compare diagnostic test performance. A wean-to-finish longitudinal study was also carried out to demonstrate the reproducibility of using pen-based oral fluids. Overall, pen-based oral and tonsillar fluids were more likely to be positive for both types of bacteria whereas only M. hyorhinis was detected in nasal fluids. DNA extraction protocols were shown to significantly influence test result. Although the initial detection time somewhat differed, both organisms were repeatedly detected in the longitudinal study. Overall, this study evaluated two qPCR methods for rapid and specific detection of either mycoplasma. Results from the present investigation can serve as a foundation for future studies to determine the prevalence of the two microorganisms, environmental load, and effectiveness of veterinary interventions for infection control.
MeSH Terms
-
Animals
Diagnostic Tests, Routine/methods/*veterinary
Female
Longitudinal Studies
Mouth/microbiology
Mycoplasma Infections/diagnosis/microbiology/*veterinary
Mycoplasma hyorhinis/*isolation & purification
Mycoplasma hyosynoviae/*isolation & purification
Nose/microbiology
Palatine Tonsil/microbiology
Real-Time Polymerase Chain Reaction/*veterinary
Reproducibility of Results
Swine
Swine Diseases/*diagnosis/microbiology
Figure
Cited by 1 articles
-
Two clinical isolates of Mycoplasma hyosynoviae showed differing pattern of lameness and pathogen detection in experimentally challenged pigs
João Carlos Gomes-Neto, Matthew Raymond, Leslie Bower, Alejandro Ramirez, Darin M. Madson, Erin L. Strait, Everett L. Rosey, Vicki J. Rapp-Gabrielson
J Vet Sci. 2016;17(4):489-496. doi: 10.4142/jvs.2016.17.4.489.
Reference
-
1. Bender JS, Shen HG, Irwin CK, Schwartz KJ, Opriessnig T. Characterization of Erysipelothrix species isolates from clinically affected pigs, environmental samples, and vaccine strains from six recent swine erysipelas outbreaks in the United States. Clin Vaccine Immunol. 2010; 17:1605–1611.
Article2. Chittick WA, Stensland WR, Prickett JR, Strait EL, Harmon K, Yoon KJ, Wang C, Zimmerman JJ. Comparison of RNA extraction and real-time reverse transcription polymerase chain reaction methods for the detection of Porcine reproductive and respiratory syndrome virus in porcine oral fluid specimens. J Vet Diagn Invest. 2011; 23:248–253.
Article3. Clavijo MJ, Oliveira S, Zimmerman J, Rendahl A, Rovira A. Field evaluation of a quantitative polymerase chain reaction assay for Mycoplasma hyorhinis. J Vet Diagn Invest. 2014; 26:755–760.
Article4. Cole JR, Wang Q, Fish JA, Chai B, McGarrell DM, Sun Y, Brown CT, Porras-Alfaro A, Kuske CR, Tiedje JM. Ribosomal database project: data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014; 42:D633–D642.
Article5. Costa G, Oliveira S, Torrison J. Detection of Actinobacillus pleuropneumoniae in oral-fluid samples obtained from experimentally infected pigs. J Swine Health Prod. 2012; 20:78–81.6. Detmer SE, Patnayak DP, Jiang Y, Gramer MR, Goyal SM. Detection of Influenza A virus in porcine oral fluid samples. J Vet Diagn Invest. 2011; 23:241–247.
Article7. Friis NF. Mycoplasmas cultivated from the respiratory tract of Danish pigs. Acta Vet Scand. 1971; 12:69–79.8. Friis NF, Ahrens P, Larsen H. Mycoplasma hyosynoviae isolation from the upper respiratory tract and tonsils of pigs. Acta Vet Scand. 1991; 32:425–429.
Article9. Gois M, Cerný M, Rozkosný V, Sovadina M. Studies on the epizootiological significance of some species of mycoplasma isolated from nasal swabs and lungs of pigs. Zentralbl Veterinarmed B. 1969; 16:253–265.
Article10. Gois M, Pospisil Z, Cerny M, Mrva V. Production of pneumonia after intranasal inoculation of gnotobiotic piglets with three strains of Mycoplasma hyorhinis. J Comp Pathol. 1971; 81:401–410.11. Gomes Neto JC, Gauger PC, Strait EL, Boyes N, Madson DM, Schwartz KJ. Mycoplasma-associated arthritis: critical points for diagnosis. J Swine Health Prod. 2012; 20:82–86.12. Gomes Neto JC, Strait EL, Raymond M, Ramirez A, Minion FC. Antibody responses of swine following infection with Mycoplasma hyopneumoniae, M. hyorhinis, M. hyosynoviae and M. flocculare. Vet Microbiol. 2014; 174:163–171.13. Hagedorn-Olsen T, Nielsen NC, Friis NF. Induction of arthritis with Mycoplasma hyosynoviae in pigs: clinical response and re-isolation of the organism from body fluids and organs. Zentralbl Veterinarmed A. 1999; 46:317–325.
Article14. Hagedorn-Olsen T, Nielsen NC, Friis NF, Nielsen J. Progression of Mycoplasma hyosynoviae infection in three pig herds. Development of tonsillar carrier state, arthritis, and antibodies in serum and synovial fluid in pigs from birth to slaughter. Zentralbl Veterinarmed A. 1999; 46:555–564.15. Harris DL, Ross RF, Switzer WP. Incidence of certain microorganisms in nasal cavities of swine in Iowa. Am J Vet Res. 1969; 30:1621–1624.16. Kawamura S, Yamamoto K, Ogata M. Isolation of Mycoplasma hyosynoviae and other mycoplasmas from the respiratory tracts of pigs by aerobic and anaerobic cultivation. Nihon Juigaku Zasshi. 1982; 44:811–814.
Article17. Kobisch M, Friis NF. Swine mycoplasmoses. Rev Sci Tech. 1996; 15:1569–1605.
Article18. Lauerman LH. Nucleic Acid Amplification Assays for Diagnosis of Animal Diseases. In : Lauerman LH, editor. Mycoplasma PCR assays. Auburn: American Association of Veterinary Laboratory Diagnostics;1998. p. 41–42.19. Lauritsen KT, Hagedorn-Olsen T, Friis NF, Lind P, Jungersen G. Absence of strictly age-related resistance to Mycoplasma hyosynoviae infection in 6-week-old pigs. Vet Microbiol. 2008; 130:385–390.
Article20. Lee ZM, Bussema C 3rd, Schmidt TM. rrnDB: documenting the number of rRNA and tRNA genes in bacteria and archaea. Nucleic Acids Res. 2009; 37:D489–D493.21. Lin JH, Chen SP, Yeh KS, Weng CN. Mycoplasma hyorhinis in Taiwan: diagnosis and isolation of swine pneumonia pathogen. Vet Microbiol. 2006; 115:111–116.
Article22. Lowe BA, Marsh TL, Isaacs-Cosgrove N, Kirkwood RN, Kiupel M, Mulks MH. Microbial communities in the tonsils of healthy pigs. Vet Microbiol. 2011; 147:346–357.
Article23. Loy A, Arnold R, Tischler P, Rattei T, Wagner M, Horn M. probeCheck - a central resource for evaluating oligonucleotide probe coverage and specificity. Environ Microbiol. 2008; 10:2894–2898.
Article24. Makhanon M, Tummaruk P, Thongkamkoon P, Thanawongnuwech R, Prapasarakul N. Comparison of detection procedures of Mycoplasma hyopneumoniae, Mycoplasma hyosynoviae, and Mycoplasma hyorhinis in lungs, tonsils, and synovial fluid of slaughtered pigs and their distributions on Thailand. Trop Anim Health Prod. 2012; 44:313–318.
Article25. Nielsen EO, Nielsen NC, Friis NF. Mycoplasma hyosynoviae arthritis in grower-finisher pigs. J Vet Med A Physiol Pathol Clin Med. 2001; 48:475–486.26. Palzer A, Ritzmann M, Wolf G, Heinritzi K. Associations between pathogens in healthy pigs and pigs with pneumonia. Vet Rec. 2008; 162:267–271.
Article27. Prickett J, Simer R, Christopher-Hennings J, Yoon KJ, Evans RB, Zimmerman JJ. Detection of porcine reproductive and respiratory syndrome virus infection in porcine oral fluids samples: a longitudinal study under experimental conditions. J Vet Diagn Invest. 2008; 20:156–163.
Article28. Prickett JR, Johnson J, Murtaugh MP, Puvanendiran S, Wang C, Zimmerman JJ, Opriessnig T. Prolonged detection of PCV2 and anti-PCV2 antibody in oral fluids following experimental inoculation. Transbound Emerg Dis. 2011; 58:121–127.
Article29. Ross RF. Predisposing factors in Mycoplasma hyosynoviae arthritis in swine. J Infect Dis. 1973; 127:Suppl. S84–S86.30. Ross RF, Duncan JR. Mycoplasma hyosynoviae arthritis of swine. J Am Vet Med Assoc. 1970; 157:1515–1518.31. Ross RF, Karmon JA. Heterogeneity among strains of Mycoplasma granularum and identification of Mycoplasma hyosynoviae, sp. n. J Bacteriol. 1970; 103:707–713.
Article32. Ross RF, Spear ML. Role of the sow as a reservoir of infection for Mycoplasma hyosynoviae. Am J Vet Res. 1973; 34:373–378.33. Ross RF, Switzer WP, Duncan JR. Experimental production of Mycoplasma hyosynoviae arthritis in swine. Am J Vet Res. 1971; 32:1743–1749.34. Strait EL, Madsen ML, Minion FC, Christopher-Hennings J, Dammen M, Jones KR, Thacker EL. Real-time PCR assays to address genetic diversity among strains of Mycoplasma hyopneumoniae. J Clin Microbiol. 2008; 46:2491–2498.
Article35. Thacker EL, Minion FC. Diseases of Swine. In : Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, editors. Mycoplasmosis. 10th ed. Ames: Wiley-Blackwell;2012. p. 779–797.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis of Mycoplasma hyorhinis infection in pigs by PCR amplification of 16S-23S rRNA internal transcribed spacer region
- An improved multiplex PCR for diagnosis and differentiation of Mycoplasma hyopneumoniae and Mycoplasma hyorhinis
- Detection of Mycoplasma pneumoriae in Clinical Samples from Pediatric Patients by Polymerase Chain Reaction
- In vitro antibiotic susceptibility of field isolates of Mycoplasma hyopneumoniae and Mycoplasma hyorhinis from Korea
- Sensitive and Specific Detection of Mycoplasma species by Consensus Polymerase Chain Reaction and Dot Blot Hybridization