Chonnam Med J.
2018 May;54(2):113-120. 10.4068/cmj.2018.54.2.113.
Role of Red Cell Distribution Width in the Relationship between Clinical Outcomes and Anticoagulation Response in Patients with Atrial Fibrillation
- Affiliations
-
- 1Department of Cardiology, Chonnam National University Hospital, Gwangju, Korea. drgood2@empas.com
- KMID: 2411894
- DOI: http://doi.org/10.4068/cmj.2018.54.2.113
Abstract
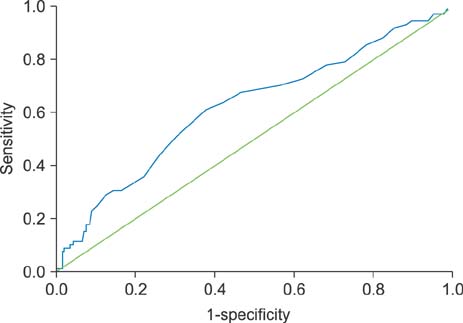
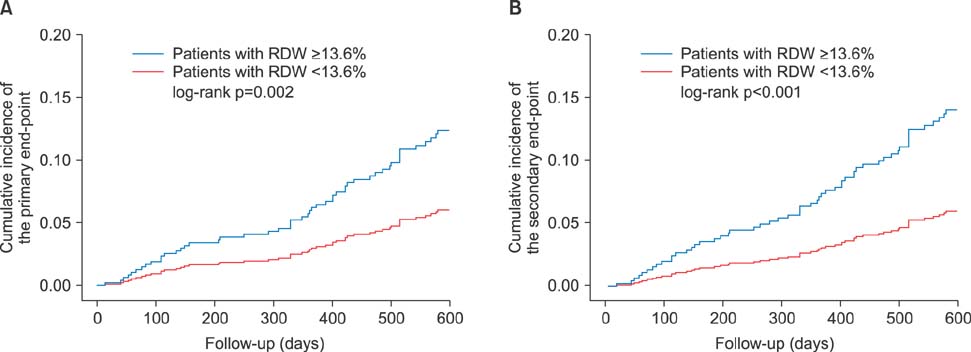
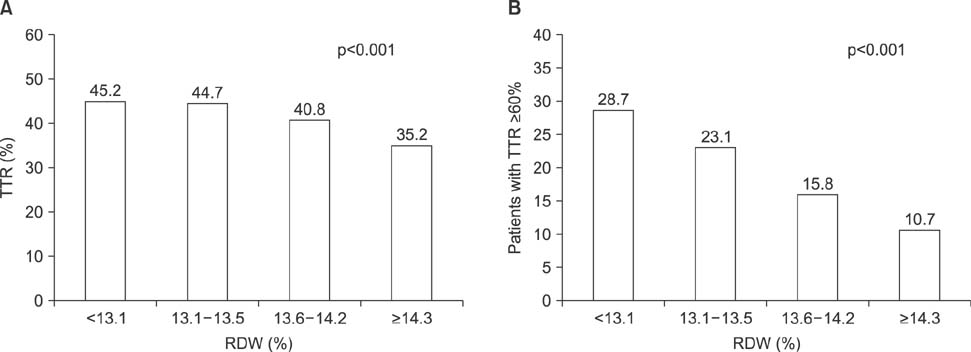
- Little is known as to why elevated red cell distribution width (RDW) is associated with adverse clinical outcomes in patients with atrial fibrillation (AF). We hypothesized that RDW value might predict the intensity of anticoagulation, resulting in higher adverse events in patients with AF taking warfarin. We analyzed 657 patients with non-valuvular AF who took warfarin. The intensity of anticoagulation was assessed as mean time in the therapeutic range (TTR) and defined TTR ≥60% as an optimal intensity. The primary end-point was the composite of stroke/systemic embolism and major bleeding. The secondary end-point was the composite of stroke/systemic embolism, major bleeding and death. The relationship between the baseline RDW with TTR and clinical outcomes was assessed using categorical variables as quartiles or dichotomous variables. The mean value of TTR decreased as an increment of the RDW (45.2% vs. 44.7% vs. 40.8% vs. 35.2%, p < 0.001). Primary and secondary end-points were significantly increased when TTR was less than 60% and RDW was more than 13.6%. Ratio of patients achieving optimal anticoagulation were significantly decreased as an increment of RDW. A RDW of ≥13.6% was a significant predictor for poor anticoagulation control (adjusted Odds ratio [OR] 0.43, 95% confidence interval [CI] 0.23-0.82), stroke (adjusted hazard ratio [HR] 3.86, 95% CI 1.11-13.40), primary (adjusted HR 1.88, 95% CI 1.12-3.16) and secondary end-point (adjusted HR 2.46, 95% CI 1.26-4.81). RDW was negatively associated with TTR in patients with AF. Therefore, RDW might be a useful marker for the prediction of anticoagulation response and clinical outcomes in patients with AF.
Keyword
MeSH Terms
Figure
Reference
-
1. Balta S, Aydogan M, Kurt O, Karaman M, Demirkol S, Akgul EO. Red cell distribution width as a novel, simple, inexpensive predictor of mortality in patients with chronic heart failure. Int J Cardiol. 2013; 168:3049–3050.
Article2. Felker GM, Allen LA, Pocock SJ, Shaw LK, McMurray JJ, Pfeffer MA, et al. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. J Am Coll Cardiol. 2007; 50:40–47.
Article3. Tonelli M, Sacks F, Arnold M, Moye L, Davis B, Pfeffer M. for the Cholesterol and Recurrent Events (CARE) Trial Investigators. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation. 2008; 117:163–168.
Article4. Sangoi MB, Da Silva SH, da Silva JE, Moresco RN. Relation between red blood cell distribution width and mortality after acute myocardial infarction. Int J Cardiol. 2011; 146:278–280.
Article5. Perlstein TS, Weuve J, Pfeffer MA, Beckman JA. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med. 2009; 169:588–594.
Article6. Adamsson Eryd S, Borné Y, Melander O, Persson M, Smith JG, Hedblad B, et al. Red blood cell distribution width is associated with incidence of atrial fibrillation. J Intern Med. 2014; 275:84–92.
Article7. Saliba W, Barnett-Griness O, Elias M, Rennert G. The association between red cell distribution width and stroke in patients with atrial fibrillation. Am J Med. 2015; 128:192.e11–192.e18.
Article8. Lee KH, Park HW, Cho JG, Yoon NS, Kim SS, Kim MR, et al. Red cell distribution width as a novel predictor for clinical outcomes in patients with paroxysmal atrial fibrillation. Europace. 2015; 17:Suppl 2. ii83–ii88.
Article9. Rosendaal FR, Cannegieter SC, van der Meer FJ, Briët E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993; 69:236–239.
Article10. Cabral KP, Ansell J, Hylek EM. Future directions of stroke prevention in atrial fibrillation: the potential impact of novel anticoagulants and stroke risk stratification. J Thromb Haemost. 2011; 9:441–449.
Article11. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016; 37:2893–2962.
Article12. Li H, Liu T, Xu G, Liu E, Jiao Z, Li G. Red blood cell distribution width and the recurrence of atrial fibrillation after ablation in patients with paroxysmal non-valvular symptomatic atrial fibrillation. Int J Cardiol. 2016; 203:834–836.
Article13. Benedetto U, Angeloni E, Melina G, Pisano C, Lechiancole A, Roscitano A, et al. Red blood cell distribution width predicts mortality after coronary artery bypass grafting. Int J Cardiol. 2013; 165:369–371.
Article14. Wan H, Yang Y, Zhu J, Huang B, Wang J, Wu S, et al. The relationship between elevated red cell distribution width and long-term outcomes among patients with atrial fibrillation. Clin Biochem. 2015; 48:762–767.
Article15. Kurt M, Tanboga IH, Buyukkaya E, Karakas MF, Akçay AB, Sen N. Relation of red cell distribution width with CHA2DS2-VASc score in patients with nonvalvular atrial fibrillation. Clin Appl Thromb Hemost. 2014; 20:687–692.
Article16. Ani C, Ovbiagele B. Elevated red blood cell distribution width predicts mortality in persons with known stroke. J Neurol Sci. 2009; 277:103–108.
Article17. Tanindi A, Topal FE, Topal F, Celik B. Red cell distribution width in patients with prehypertension and hypertension. Blood Press. 2012; 21:177–181.
Article18. Sánchez-Chaparro MA, Calvo-Bonacho E, González-Quintela A, Cabrera M, Sáinz JC, Fernández-Labandera C, et al. Higher red blood cell distribution width is associated with the metabolic syndrome: results of the Ibermutuamur CArdiovascular RIsk assessment study. Diabetes Care. 2010; 33:e40.
Article19. Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012; 60:2263–2270.
Article20. Rodríguez-Carrio J, Alperi-López M, López P, Alonso-Castro S, Carro-Esteban SR, Ballina-García FJ, et al. Red cell distribution width is associated with endothelial progenitor cell depletion and vascular-related mediators in rheumatoid arthritis. Atherosclerosis. 2015; 240:131–136.
Article21. Solak Y, Yilmaz MI, Saglam M, Caglar K, Verim S, Unal HU, et al. Red cell distribution width is independently related to endothelial dysfunction in patients with chronic kidney disease. Am J Med Sci. 2014; 347:118–124.
Article22. Demirkol S, Balta S, Celik T, Arslan Z, Unlu M, Cakar M, et al. Assessment of the relationship between red cell distribution width and cardiac syndrome X. Kardiol Pol. 2013; 71:480–484.
Article23. Stern DM, Esposito C, Gerlach H, Gerlach M, Ryan J, Handley D, et al. Endothelium and regulation of coagulation. Diabetes Care. 1991; 14:160–166.
Article24. Preissner KT. Anticoagulant potential of endothelial cell membrane components. Haemostasis. 1988; 18:271–300.
Article25. Cho HJ, Kim DW, Kim GS, Jeong IS. Anticoagulation therapy during extracorporeal membrane oxygenator support in pediatric patients. Chonnam Med J. 2017; 53:110–117.
Article26. Lee J, Bae EH, Ma SK, Kim SW. Altered nitric oxide system in cardiovascular and renal diseases. Chonnam Med J. 2016; 52:81–90.
Article27. Hatami M, Saidijam M, Yadegarzari R, Borzuei S, Soltanian A, Arian MS, et al. Peroxisome Proliferator-Activated Receptor-γ Gene Expression and Its Association with Oxidative Stress in Patients with Metabolic Syndrome. Chonnam Med J. 2016; 52:201–206.
Article28. Balta S, Demir M, Kucuk U, Arslan Z, Demirkol S. Red cell distribution width in patients with atrial fibrillation. J Intern Med. 2014; 275:545.
Article29. Förhécz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohászka Z, Jánoskuti L. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J. 2009; 158:659–666.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- How and When to Screen for Atrial Fibrillation after Stroke: Insights from Insertable Cardiac Monitoring Devices
- The Mechanism of and Preventive Therapy for Stroke in Patients with Atrial Fibrillation
- Cardioembolic Stroke in Atrial Fibrillation-Rationale for Preventive Closure of the Left Atrial Appendage
- Anticoagulation in Atrial Fibrillation
- Stroke Prevention in Atrial Fibrillation