Korean J Radiol.
2018 Jun;19(3):481-488. 10.3348/kjr.2018.19.3.481.
Prognostic Value of Baseline ¹â¸F-Fluorodeoxyglucose PET/CT in Patients with Multiple Myeloma: A Multicenter Cohort Study
- Affiliations
-
- 1Department of Nuclear Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 06351, Korea. jynm.choi@samsung.com
- 2Department of Radiology, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Suwon 16247, Korea.
- 3Department of Radiology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea.
- 4Department of Nuclear Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul 03080, Korea.
- 5Department of Nuclear Medicine, Kyungpook National University Medical Center, Kyungpook National University School of Medicine, Daegu 41566, Korea.
- 6Department of Medicine, Division of Hematology-Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 06351, Korea.
- KMID: 2410819
- DOI: http://doi.org/10.3348/kjr.2018.19.3.481
Abstract
OBJECTIVE
We investigated the prognostic value of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) in patients with multiple myeloma (MM).
MATERIALS AND METHODS
Subjects were 76 patients with newly diagnosed myeloma and pretreatment with 18F-FDG PET/CT from four hospitals. The PET/CT features were evaluated and the clinical characteristics were reviewed. Prognostic factors related to poor progression-free survival (PFS) and overall survival (OS) were identified using a Cox proportional hazards regression model and a prediction scale was developed based on the identified factors.
RESULTS
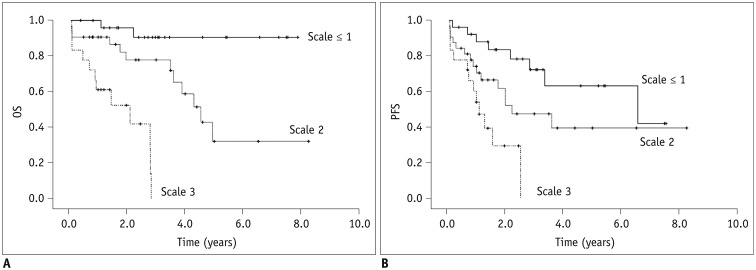
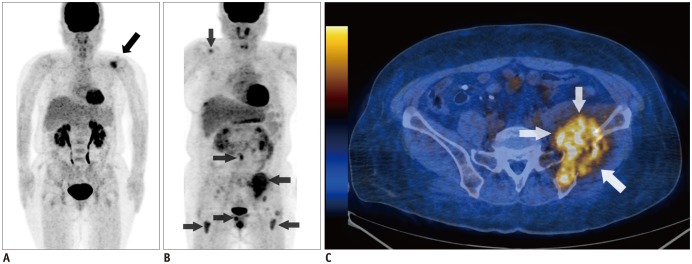
Multivariate analysis showed that the presence of 18F-FDG-avid focal bone lesions (≥ 3) was a significant and independent predictor of PFS (hazard ratio [HR] = 3.28, p = 0.007) and OS (HR = 11.78, p = 0.001). The presence of extramedullary disease on PET/CT scan was also a significant predictor of poor PFS (HR = 2.79, p = 0.006) and OS (HR = 3.89, p = 0.003). A prognostic scale was developed using these two predictors. An increase in score on the scale corresponded to a significantly increased risk of poor OS (p = 0.005). In addition, Kaplan-Meier analysis demonstrated that patient survival varied significantly according to the scale (p < 0.001 for OS and p = 0.001 for PFS).
CONCLUSION
18F-FDG-avid focal lesions and the presence of extramedullary disease on PET/CT scan are significantly associated with poor OS in MM patients. The scale developed according to these predictors represents a potential prognostic tool for evaluation of patients with MM.
Keyword
MeSH Terms
Figure
Reference
-
1. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011; 364:1046–1060. PMID: 21410373.
Article2. Terpos E, Dimopoulos MA. Myeloma bone disease: pathophysiology and management. Ann Oncol. 2005; 16:1223–1231. PMID: 15928069.
Article3. Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003; 78:21–33. PMID: 12528874.
Article4. Dimopoulos M, Terpos E, Comenzo RL, Tosi P, Beksac M, Sezer O, et al. International myeloma working group consensus statement and guidelines regarding the current role of imaging techniques in the diagnosis and monitoring of multiple myeloma. Leukemia. 2009; 23:1545–1556. PMID: 19421229.
Article5. Zamagni E, Cavo M. The role of imaging techniques in the management of multiple myeloma. Br J Haematol. 2012; 159:499–513. PMID: 22881361.
Article6. Delorme S, Baur-Melnyk A. Imaging in multiple myeloma. Eur J Radiol. 2009; 70:401–408. PMID: 19272726.
Article7. Adam Z, Bolcak K, Stanicek J, Buchler T, Pour L, Krejci M, et al. Fluorodeoxyglucose positron emission tomography in multiple myeloma, solitary plasmocytoma and monoclonal gammapathy of unknown significance. Neoplasma. 2007; 54:536–540. PMID: 17949238.8. Zamagni E, Nanni C, Patriarca F, Englaro E, Castellucci P, Geatti O, et al. A prospective comparison of 18F-fluorodeoxyglucose positron emission tomographycomputed tomography, magnetic resonance imaging and whole-body planar radiographs in the assessment of bone disease in newly diagnosed multiple myeloma. Haematologica. 2007; 92:50–55. PMID: 17229635.
Article9. Agarwal A, Chirindel A, Shah BA, Subramaniam RM. Evolving role of FDG PET/CT in multiple myeloma imaging and management. AJR Am J Roentgenol. 2013; 200:884–890. PMID: 23521465.
Article10. Dinter DJ, Neff WK, Klaus J, Bőhm C, Hastka J, Weiss C, et al. Comparison of whole-body MR imaging and conventional X-ray examination in patients with multiple myeloma and implications for therapy. Ann Hematol. 2009; 88:457–464. PMID: 18941746.
Article11. Ghanem N, Uhl M, Brink I, Schäfer O, Kelly T, Moser E, et al. Diagnostic value of MRI in comparison to scintigraphy, PET, MS-CT and PET/CT for the detection of metastases of bone. Eur J Radiol. 2005; 55:41–55. PMID: 15950100.
Article12. Bartel TB, Haessler J, Brown TL, Shaughnessy JD Jr, van Rhee F, Anaissie E, et al. F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood. 2009; 114:2068–2076. PMID: 19443657.
Article13. Zamagni E, Patriarca F, Nanni C, Zannetti B, Englaro E, Pezzi A, et al. Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood. 2011; 118:5989–5995. PMID: 21900189.
Article14. Dimitrakopoulou-Strauss A, Hoffmann M, Bergner R, Uppenkamp M, Haberkorn U, Strauss LG. Prediction of progression-free survival in patients with multiple myeloma following anthracycline-based chemotherapy based on dynamic FDG-PET. Clin Nucl Med. 2009; 34:576–584. PMID: 19692817.15. Haznedar R, Aki SZ, Akdemir OU, Ozkurt ZN, Ceneli O, Yağcı M, et al. Value of 18F-fluorodeoxyglucose uptake in positron emission tomography/computed tomography in predicting survival in multiple myeloma. Eur J Nucl Med Mol Imaging. 2011; 38:1046–1053. PMID: 21287167.
Article16. Park S, Lee SJ, Chang WJ, Maeng CH, Hong JY, Choi MK, et al. Positive correlation between baseline PET or PET/CT findings and clinical parameters in multiple myeloma patients. Acta Haematol. 2014; 131:193–199. PMID: 24296366.
Article17. Rajan AM, Rajkumar SV. Interpretation of cytogenetic results in multiple myeloma for clinical practice. Blood Cancer J . 2015; 5:e365. PMID: 26517360.
Article18. Pour L, Sevcikova S, Greslikova H, Kupska R, Majkova P, Zahradova L, et al. Soft-tissue extramedullary multiple myeloma prognosis is significantly worse in comparison to bone-related extramedullary relapse. Haematologica. 2014; 99:360–364. PMID: 24038024.
Article19. Usmani SZ, Heuck C, Mitchell A, Szymonifka J, Nair B, Hoering A, et al. Extramedullary disease portends poor prognosis in multiple myeloma and is over-represented in high-risk disease even in the era of novel agents. Haematologica. 2012; 97:1761–1767. PMID: 22689675.
Article20. Oriol A. Multiple myeloma with extramedullary disease. Adv Ther. 2011; 28(7):1–6.
Article21. Varettoni M, Corso A, Pica G, Mangiacavalli S, Pascutto C, Lazzarino M. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann Oncol. 2010; 21:325–330. PMID: 19633044.
Article22. Soret M, Bacharach SL, Buvat I. Partial-volume effect in PET tumor imaging. J Nucl Med. 2007; 48:932–945. PMID: 17504879.
Article23. Kim SJ, Yi HK, Lim CH, Cho YS, Choi JY, Choe YS, et al. Intra-patient variability of FDG standardized uptake values in mediastinal blood pool, liver, and myocardium during R-CHOP chemotherapy in patients with diffuse large B-cell lymphoma. Nucl Med Mol Imaging. 2016; 50:300–307. PMID: 27994685.
Article24. Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008; 111:2516–2520. PMID: 17975015.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- â¶â¸Gallium-Arginine-Glycine-Aspartic Acid and ¹â¸F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Chondroblastic Osteosarcoma of the Skull
- Dual Pathologies of Parathyroid Adenoma and Papillary Thyroid Cancer on Fluorocholine and Fluorodeoxyglucose PET/CT
- Role of ¹â¸F-FDG PET-CT in Monitoring the Cyclophosphamide Induced Pulmonary Toxicity in Patients with Breast Cancer: 2 Case Reports
- Skin Manifestation of Unsuspecting Prostate Cancer Detected by 18F-FDG PET/CT Performed To Assess Underlying Multiple Myeloma
- Primary Renal Leiomyosarcoma Presenting with Subcutaneous and Osseous Metastases: Staging and Follow-Up with 18F-FDG PET/CT