Endocrinol Metab.
2015 Mar;30(1):53-57. 10.3803/EnM.2015.30.1.53.
Determination of Mother Centriole Maturation in CPAP-Depleted Cells Using the Ninein Antibody
- Affiliations
-
- 1Department of Biological Sciences, Seoul National University, Seoul, Korea. rheek@snu.ac.kr
- KMID: 2407104
- DOI: http://doi.org/10.3803/EnM.2015.30.1.53
Abstract
- BACKGROUND
Mutations in centrosomal protein genes have been identified in a number of genetic diseases in brain development, including microcephaly. Centrosomal P4.1-associated protein (CPAP) is one of the causal genes implicated in primary microcephaly. We previously proposed that CPAP is essential for mother centriole maturation during mitosis.
METHODS
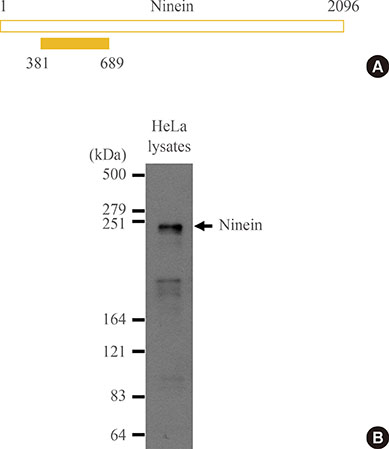
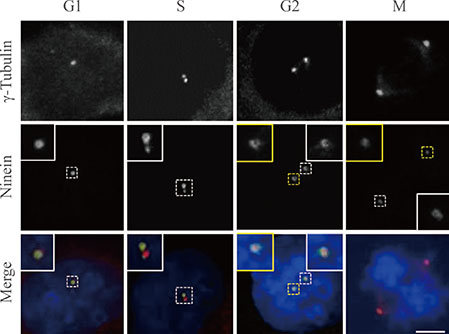
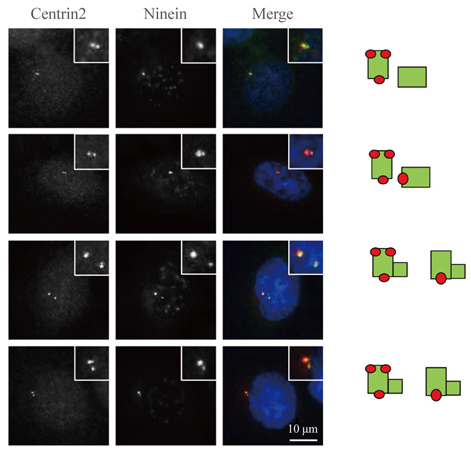
We immunostained CPAP-depleted cells using the ninein antibody, which selectively detects subdistal appendages in mature mother centrioles.
RESULTS
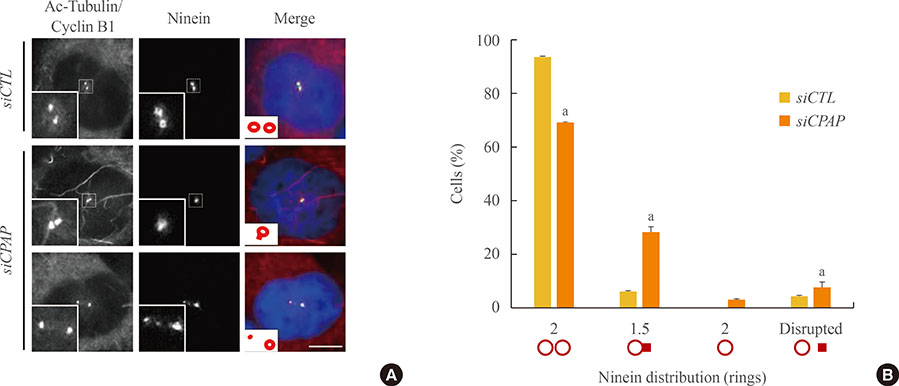
Ninein signals were significantly impaired in CPAP-depleted cells.
CONCLUSION
The results suggest that CPAP is required for mother centriole maturation in mammalian cells. The selective absence of centriolar appendages in young mother centrioles may be responsible for asymmetric spindle pole formation in CPAP-depleted cells.
Keyword
Figure
Reference
-
1. Tsou MF, Stearns T. Mechanism limiting centrosome duplication to once per cell cycle. Nature. 2006; 442:947–951.2. Mardin BR, Schiebel E. Breaking the ties that bind: new advances in centrosome biology. J Cell Biol. 2012; 197:11–18.3. Loncarek J, Khodjakov A. Ab ovo or de novo? Mechanisms of centriole duplication. Mol Cells. 2009; 27:135–142.4. Thornton GK, Woods CG. Primary microcephaly: do all roads lead to Rome? Trends Genet. 2009; 25:501–510.5. Bond J, Roberts E, Springell K, Lizarraga SB, Scott S, Higgins J, Hampshire DJ, Morrison EE, Leal GF, Silva EO, Costa SM, Baralle D, Raponi M, Karbani G, Rashid Y, Jafri H, Bennett C, Corry P, Walsh CA, Woods CG. A centrosomal mechanism involving CDK5RAP2 and CENPJ controls brain size. Nat Genet. 2005; 37:353–355.6. Lee M, Chang J, Chang S, Lee KS, Rhee K. Asymmetric spindle pole formation in CPAP-depleted mitotic cells. Biochem Biophys Res Commun. 2014; 444:644–650.7. Bouckson-Castaing V, Moudjou M, Ferguson DJ, Mucklow S, Belkaid Y, Milon G, Crocker PR. Molecular characterisation of ninein, a new coiled-coil protein of the centrosome. J Cell Sci. 1996; 109(Pt 1):179–190.8. Chen CH, Howng SL, Cheng TS, Chou MH, Huang CY, Hong YR. Molecular characterization of human ninein protein: two distinct subdomains required for centrosomal targeting and regulating signals in cell cycle. Biochem Biophys Res Commun. 2003; 308:975–983.9. Mogensen MM, Malik A, Piel M, Bouckson-Castaing V, Bornens M. Microtubule minus-end anchorage at centrosomal and non-centrosomal sites: the role of ninein. J Cell Sci. 2000; 113(Pt 17):3013–3023.10. Chang J, Cizmecioglu O, Hoffmann I, Rhee K. PLK2 phosphorylation is critical for CPAP function in procentriole formation during the centrosome cycle. EMBO J. 2010; 29:2395–2406.11. Cheng TS, Hsiao YL, Lin CC, Hsu CM, Chang MS, Lee CI, Yu RC, Huang CY, Howng SL, Hong YR. hNinein is required for targeting spindle-associated protein Astrin to the centrosome during the S and G2 phases. Exp Cell Res. 2007; 313:1710–1721.12. Ou YY, Mack GJ, Zhang M, Rattner JB. CEP110 and ninein are located in a specific domain of the centrosome associated with centrosome maturation. J Cell Sci. 2002; 115(Pt 9):1825–1835.13. Delgehyr N, Sillibourne J, Bornens M. Microtubule nucleation and anchoring at the centrosome are independent processes linked by ninein function. J Cell Sci. 2005; 118(Pt 8):1565–1575.14. Wang X, Tsai JW, Imai JH, Lian WN, Vallee RB, Shi SH. Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature. 2009; 461:947–955.15. Shinohara H, Sakayori N, Takahashi M, Osumi N. Ninein is essential for the maintenance of the cortical progenitor character by anchoring the centrosome to microtubules. Biol Open. 2013; 2:739–749.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Respiratory Effects of Continuous Flow CPAP System with Demand Flow CPAP System
- The Effets of Independent Lung Ventilation with Unilateral HFJV and CPAP
- Maturation of Stem Cell-Derived Cardiomyocytes: Foe in Translation Medicine
- Is There Any Difference in Arterial Oxygenation between the Right and Left Thoracic Surgery under the Different One Lung Ventilation Mode?
- The compliance and effect of CPAP in Obstructive Sleep Apnea Syndrome