Korean J Radiol.
2018 Apr;19(2):358-365. 10.3348/kjr.2018.19.2.358.
A Whole-Tumor Histogram Analysis of Apparent Diffusion Coefficient Maps for Differentiating Thymic Carcinoma from Lymphoma
- Affiliations
-
- 1Department of Radiology, The First Affiliated Hospital of Nanjing Medical University, Nanjing 210000, China.
- 2Department of Thoracic Surgery, The First Affiliated Hospital of Nanjing Medical University, Nanjing 210000, China.
- 3Department of Nutrition and Food Hygiene, School of Public Health, Nanjing Medical University, Nanjing 211166, China. qingfeng@njmu.edu.cn
- KMID: 2404934
- DOI: http://doi.org/10.3348/kjr.2018.19.2.358
Abstract
OBJECTIVE
To assess the performance of a whole-tumor histogram analysis of apparent diffusion coefficient (ADC) maps in differentiating thymic carcinoma from lymphoma, and compare it with that of a commonly used hot-spot region-of-interest (ROI)-based ADC measurement.
MATERIALS AND METHODS
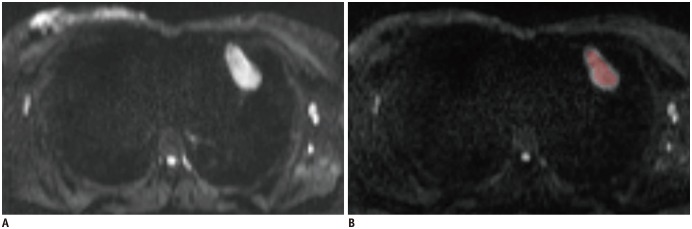
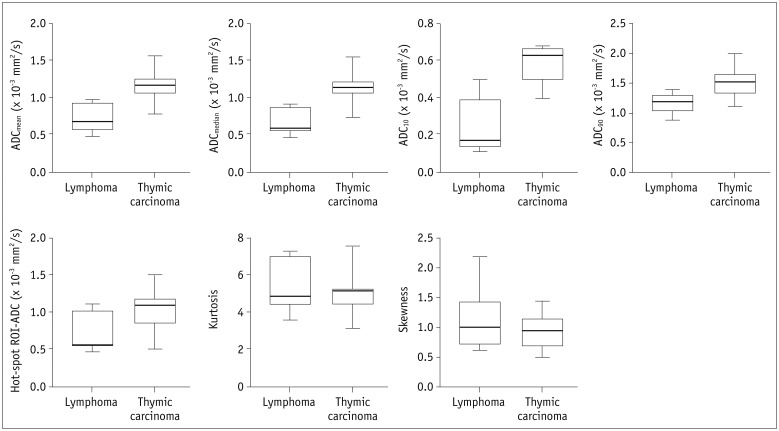
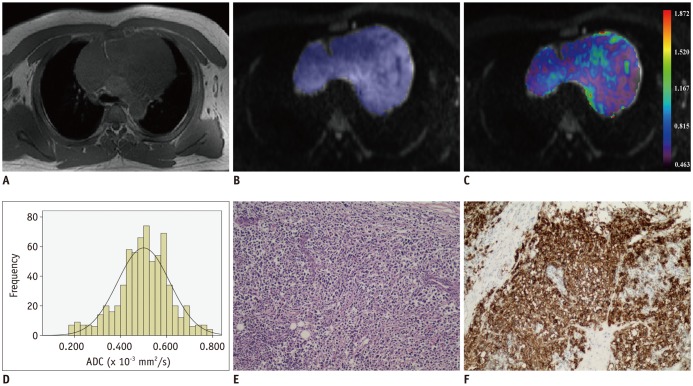
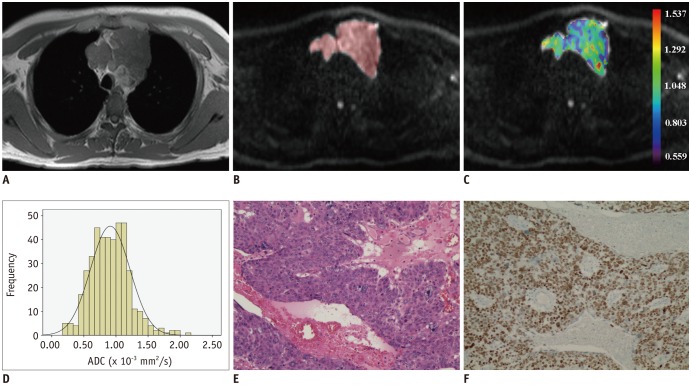
Diffusion weighted imaging data of 15 patients with thymic carcinoma and 13 patients with lymphoma were retrospectively collected and processed with a mono-exponential model. ADC measurements were performed by using a histogram-based and hot-spot-ROI-based approach. In the histogram-based approach, the following parameters were generated: mean ADC (ADCmean), median ADC (ADCmedian), 10th and 90th percentile of ADC (ADC10 and ADC90), kurtosis, and skewness. The difference in ADCs between thymic carcinoma and lymphoma was compared using a t test. Receiver operating characteristic analyses were conducted to determine and compare the differentiating performance of ADCs.
RESULTS
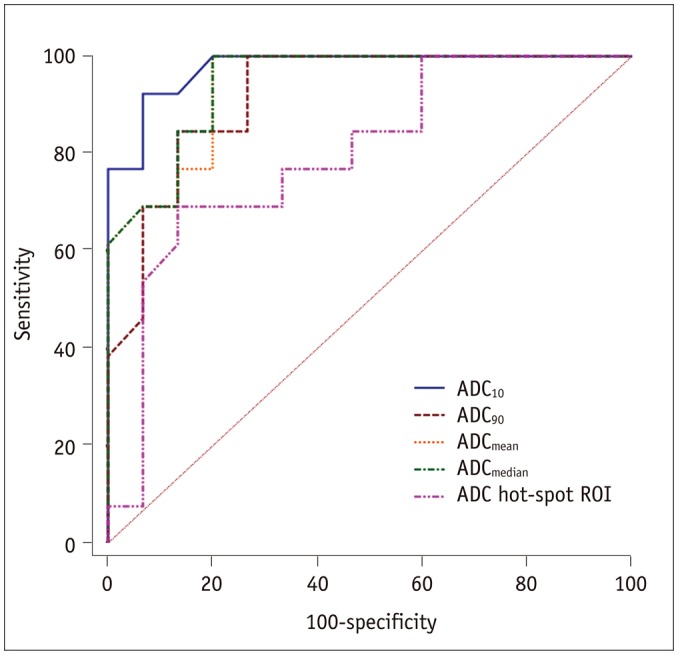
Lymphoma demonstrated significantly lower ADCmean, ADCmedian, ADC10, ADC90, and hot-spot-ROI-based mean ADC than those found in thymic carcinoma (all p values < 0.05). There were no differences found in the kurtosis (p = 0.412) and skewness (p = 0.273). The ADC10 demonstrated optimal differentiating performance (cut-off value, 0.403 × 10−3 mm2/s; area under the receiver operating characteristic curve [AUC], 0.977; sensitivity, 92.3%; specificity, 93.3%), followed by the ADCmean, ADCmedian, ADC90, and hot-spot-ROI-based mean ADC. The AUC of ADC10 was significantly higher than that of the hot spot ROI based ADC (0.977 vs. 0.797, p = 0.036).
CONCLUSION
Compared with the commonly used hot spot ROI based ADC measurement, a histogram analysis of ADC maps can improve the differentiating performance between thymic carcinoma and lymphoma.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Distinguishing between Thymic Epithelial Tumors and Benign Cysts via Computed Tomography
Sang Hyup Lee, Soon Ho Yoon, Ju Gang Nam, Hyung Jin Kim, Su Yeon Ahn, Hee Kyung Kim, Hyun Ju Lee, Hwan Hee Lee, Gi Jeong Cheon, Jin Mo Goo
Korean J Radiol. 2019;20(4):671-682. doi: 10.3348/kjr.2018.0400.Quantitative Thoracic Magnetic Resonance Criteria for the Differentiation of Cysts from Solid Masses in the Anterior Mediastinum
Eui Jin Hwang, MunYoung Paek, Soon Ho Yoon, Jihang Kim, Ho Yun Lee, Jin Mo Goo, Hyungjin Kim, Heekyung Kim, Jeanne B. Ackman
Korean J Radiol. 2019;20(5):854-861. doi: 10.3348/kjr.2018.0699.
Reference
-
1. Azizad S, Sannananja B, Restrepo CS. Solid tumors of the mediastinum in adults. Semin Ultrasound CT MR. 2016; 37:196–211. PMID: 27261345.
Article2. Shepherd A, Riely G, Detterbeck F, Simone CB 2nd, Ahmad U, Huang J, et al. Thymic Carcinoma Management Patterns among International Thymic Malignancy Interest Group (ITMIG) physicians with consensus from the Thymic Carcinoma Working Group. J Thorac Oncol. 2017; 12:745–751. PMID: 27876674.
Article3. Piña-Oviedo S, Moran CA. Primary mediastinal classical hodgkin lymphoma. Adv Anat Pathol. 2016; 23:285–309. PMID: 27441757.
Article4. Abdel Razek AA, Soliman N, Elashery R. Apparent diffusion coefficient values of mediastinal masses in children. Eur J Radiol. 2012; 81:1311–1314. PMID: 21439745.
Article5. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988; 44:837–845. PMID: 3203132.
Article6. Abdel Razek AA, Khairy M, Nada N. Diffusion-weighted MR imaging in thymic epithelial tumors: correlation with World Health Organization classification and clinical staging. Radiology. 2014; 273:268–275. PMID: 24877982.
Article7. Gümüştaş S, Inan N, Sarisoy HT, Anik Y, Arslan A, Ciftçi E, et al. Malignant versus benign mediastinal lesions: quantitative assessment with diffusion weighted MR imaging. Eur Radiol. 2011; 21:2255–2260. PMID: 21698463.
Article8. Shin KE, Yi CA, Kim TS, Lee HY, Choi YS, Kim HK, et al. Diffusion-weighted MRI for distinguishing non-neoplastic cysts from solid masses in the mediastinum: problem-solving in mediastinal masses of indeterminate internal characteristics on CT. Eur Radiol. 2014; 24:677–684. PMID: 24177751.
Article9. Seki S, Koyama H, Ohno Y, Nishio M, Takenaka D, Maniwa Y, et al. Diffusion-weighted MR imaging vs. multi-detector row CT: direct comparison of capability for assessment of management needs for anterior mediastinal solitary tumors. Eur J Radiol. 2014; 83:835–842. PMID: 24636535.
Article10. Yabuuchi H, Matsuo Y, Abe K, Baba S, Sunami S, Kamitani T, et al. Anterior mediastinal solid tumours in adults: characterisation using dynamic contrast-enhanced MRI, diffusion-weighted MRI, and FDG-PET/CT. Clin Radiol. 2015; 70:1289–1298. PMID: 26272529.11. Xu X, Su G, Hu H, Wang Y, Hong X, Shi H, et al. Effects of regions of interest methods on apparent coefficient measurement of the parotid gland in early Sjögren's syndrome at 3T MRI. Acta Radiol. 2017; 58:27–33. PMID: 26987670.
Article12. Zhang YD, Wang Q, Wu CJ, Wang XN, Zhang J, Liu H, et al. The histogram analysis of diffusion-weighted intravoxel incoherent motion (IVIM) imaging for differentiating the gleason grade of prostate cancer. Eur Radiol. 2015; 25:994–1004. PMID: 25430007.
Article13. Lu SS, Kim SJ, Kim N, Kim HS, Choi CG, Lim YM. Histogram analysis of apparent diffusion coefficient maps for differentiating primary CNS lymphomas from tumefactive demyelinating lesions. AJR Am J Roentgenol. 2015; 204:827–834. PMID: 25794073.
Article14. Xu XQ, Hu H, Su GY, Zhang L, Liu H, Hong XN, et al. Orbital indeterminate lesions in adults: combined magnetic resonance morphometry and histogram analysis of apparent diffusion coefficient maps for predicting malignancy. Acad Radiol. 2016; 23:200–208. PMID: 26625705.15. Choi MH, Oh SN, Rha SE, Choi JI, Lee SH, Jang HS, et al. Diffusion-weighted imaging: apparent diffusion coefficient histogram analysis for detecting pathologic complete response to chemoradiotherapy in locally advanced rectal cancer. J Magn Reson Imaging. 2016; 44:212–220. PMID: 26666560.
Article16. Nougaret S, Vargas HA, Lakhman Y, Sudre R, Do RK, Bibeau F, et al. Intravoxel incoherent motion-derived histogram metrics for assessment of response after combined chemotherapy and radiation therapy in rectal cancer: initial experience and comparison between single-section and volumetric analyses. Radiology. 2016; 280:446–454. PMID: 26919562.
Article17. Xu XQ, Hu H, Su GY, Liu H, Hong XN, Shi HB, et al. Utility of histogram analysis of ADC maps for differentiating orbital tumors. Diagn Interv Radiol. 2016; 22:161–167. PMID: 26829400.
Article18. Razek AA, Elkhamary S, Mousa A. Differentiation between benign and malignant orbital tumors at 3-T diffusion MR-imaging. Neuroradiology. 2011; 53:517–522. PMID: 21286695.
Article19. Takahashi K, Al-Janabi NJ. Computed tomography and magnetic resonance imaging of mediastinal tumors. J Magn Reson Imaging. 2010; 32:1325–1339. PMID: 21105138.
Article20. Donati OF, Mazaheri Y, Afaq A, Vargas HA, Zheng J, Moskowitz CS, et al. Prostate cancer aggressiveness: assessment with whole-lesion histogram analysis of the apparent diffusion coefficient. Radiology. 2014; 271:143–152. PMID: 24475824.
Article21. Suo S, Zhang K, Cao M, Suo X, Hua J, Geng X, et al. Characterization of breast masses as benign or malignant at 3.0T MRI with whole-lesion histogram analysis of the apparent diffusion coefficient. J Magn Reson Imaging. 2016; 43:894–902. PMID: 26343918.
Article22. Johnson PW, Davies AJ. Primary mediastinal B-cell lymphoma. Hematology Am Soc Hematol Educ Program. 2008; 349–358. PMID: 19074109.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- True Progression versus Pseudoprogression in the Treatment of Glioblastomas: A Comparison Study of Normalized Cerebral Blood Volume and Apparent Diffusion Coefficient by Histogram Analysis
- Clinical applications and characteristics of apparent diffusion coefficient maps for the brain of two dogs
- Diffusion-weighted Imaging and Apparent Diffusion Coefficient Maps for the Evaluation of Pyogenic Ventriculitis
- Histogram Analysis of Diffusion Kurtosis Magnetic Resonance Imaging for Diagnosis of Hepatic Fibrosis
- Utilization of Magnetic Resonance Imaging in the Diagnosis of Thymic Diseases