Korean J Radiol.
2018 Oct;19(5):916-922. 10.3348/kjr.2018.19.5.916.
Histogram Analysis of Diffusion Kurtosis Magnetic Resonance Imaging for Diagnosis of Hepatic Fibrosis
- Affiliations
-
- 1Department of Radiology, Zhongshan Hospital, Fudan University, Shanghai Institute of Medical Imaging, Shanghai 200032, China. mengsuzeng@163.com
- 2Department of Pathology, Zhongshan Hospital, Fudan University, Shanghai 200032, China.
- 3MR Collaboration NEA, Siemens Ltd. China, Shanghai 201318, China.
- KMID: 2418555
- DOI: http://doi.org/10.3348/kjr.2018.19.5.916
Abstract
OBJECTIVE
To investigate the diagnostic value of diffusion kurtosis imaging (DKI) histogram analysis in hepatic fibrosis staging.
MATERIALS AND METHODS
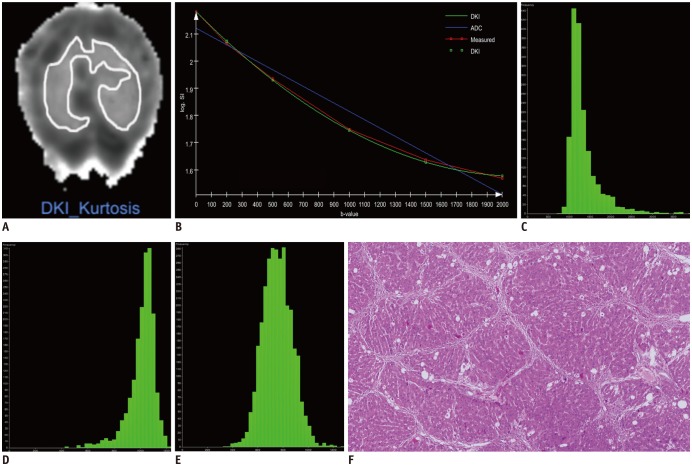
Thirty-six rats were divided into carbon tetrachloride-induced fibrosis groups (6 rats per group for 2, 4, 6, and 8 weeks) and a control group (n = 12). MRI was performed using a 3T scanner. Histograms of DKI were obtained for corrected apparent diffusion (D), kurtosis (K) and apparent diffusion coefficient (ADC). Mean, median, skewness, kurtosis and 25th and 75th percentiles were generated and compared according to the fibrosis stage and inflammatory activity.
RESULTS
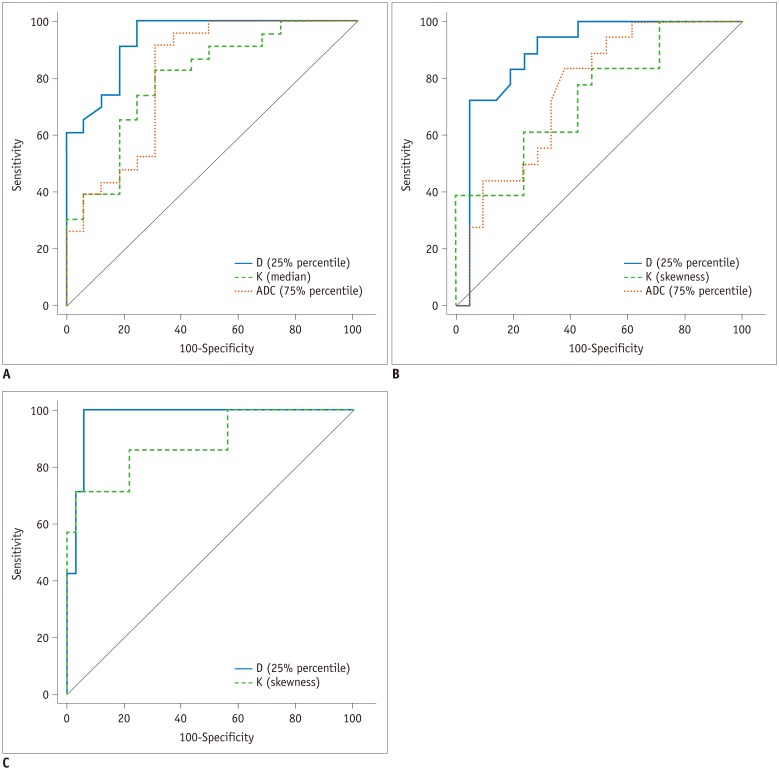
A total of 35 rats were included, and 12, 5, 5, 6, and 7 rats were diagnosed as F0-F4. The mean, median, 25th and 75th percentiles, kurtosis of D map, median, 25th percentile, skewness of K map, and 75th percentile of ADC map demonstrated significant correlation with fibrosis stage (r = −0.767 to 0.339, p < 0.001 to p = 0.039). The fibrosis score was the independent variable associated with histogram parameters compared with inflammatory activity grade (p < 0.001 to p = 0.041), except the median of K map (p = 0.185). Areas under the receiver operating characteristic curve of D were larger than K and ADC maps in fibrosis staging, although no significant differences existed in pairwise comparisons (p = 0.0512 to p = 0.847).
CONCLUSION
Corrected apparent diffusion of DKI histogram analysis provides added value and better diagnostic performance to detect various liver fibrosis stages compared with ADC.
MeSH Terms
Figure
Reference
-
1. Polasek M, Fuchs BC, Uppal R, Schühle DT, Alford JK, Loving GS, et al. Molecular MR imaging of liver fibrosis: a feasibility study using rat and mouse models. J Hepatol. 2012; 57:549–555. PMID: 22634342.
Article2. Faria SC, Ganesan K, Mwangi I, Shiehmorteza M, Viamonte B, Mazhar S, et al. MR imaging of liver fibrosis: current state of the art. Radiographics. 2009; 29:1615–1635. PMID: 19959511.
Article3. Trautwein C, Friedman SL, Schuppan D, Pinzani M. Hepatic fibrosis: concept to treatment. J Hepatol. 2015; 62(1 Suppl):S15–S24. PMID: 25920084.
Article4. Yoon JH, Lee JM, Baek JH, Shin CI, Kiefer B, Han JK, et al. Evaluation of hepatic fibrosis using intravoxel incoherent motion in diffusion-weighted liver MRI. J Comput Assist Tomogr. 2014; 38:110–116. PMID: 24378888.
Article5. Ahn SJ, Lee JM, Chang W, Lee SM, Kang HJ, Yang H, et al. Prospective validation of intra- and interobserver reproducibility of a new point shear wave elastographic technique for assessing liver stiffness in patients with chronic liver disease. Korean J Radiol. 2017; 18:926–935. PMID: 29089825.
Article6. Lee GM, Kim YR, Ryu JH, Kim TH, Cho EY, Lee YH, et al. Quantitative measurement of hepatic fibrosis with gadoxetic acid-enhanced magnetic resonance imaging in patients with chronic hepatitis B infection: a comparative study on aspartate aminotransferase to platelet ratio index and fibrosis-4 index. Korean J Radiol. 2017; 18:444–451. PMID: 28458596.
Article7. Xie S, Li Q, Cheng Y, Zhang Y, Zhuo Z, Zhao G, et al. Impact of liver fibrosis and fatty liver on T1rho measurements: a prospective study. Korean J Radiol. 2017; 18:898–905. PMID: 29089822.
Article8. Jensen JH, Helpern JA, Ramani A, Lu HZ, Kaczynski K. Diffusional kurtosis imaging: the quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005; 53:1432–1440. PMID: 15906300.
Article9. Rosenkrantz AB, Sigmund EE, Winnick A, Niver BE, Spieler B, Morgan GR, et al. Assessment of hepatocellular carcinoma using apparent diffusion coefficient and diffusion kurtosis indices: preliminary experience in fresh liver explants. Magn Reson Imaging. 2012; 30:1534–1540. PMID: 22819175.
Article10. Sheng RF, Wang HQ, Yang L, Jin KP, Xie YH, Chen CZ, et al. Diffusion kurtosis imaging and diffusion-weighted imaging in assessment of liver fibrosis stage and necroinflammatory activity. Abdom Radiol (NY). 2017; 42:1176–1182. PMID: 27866239.
Article11. Hu XX, Yang ZX, Liang HY, Ding Y, Grimm R, Fu CX, et al. Whole-tumor MRI histogram analyses of hepatocellular carcinoma: correlations with Ki-67 labeling index. J Magn Reson Imaging. 2017; 46:383–392. PMID: 27862582.
Article12. Huang YQ, Liang HY, Yang ZX, Ding Y, Zeng MS, Rao SX. Value of MR histogram analyses for prediction of microvascular invasion of hepatocellular carcinoma. Medicine (Baltimore). 2016; 95:e4034. PMID: 27368028.
Article13. Fujimoto K, Tonan T, Azuma S, Kage M, Nakashima O, Johkoh T, et al. Evaluation of the mean and entropy of apparent diffusion coefficient values in chronic hepatitis C: correlation with pathologic fibrosis stage and inflammatory activity grade. Radiology. 2011; 258:739–748. PMID: 21248235.
Article14. Choi JY, Kim H, Sun M, Sirlin CB. Histogram analysis of hepatobiliary phase MR imaging as a quantitative value for liver cirrhosis: preliminary observations. Yonsei Med J. 2014; 55:651–659. PMID: 24719131.
Article15. Kim H, Park SH, Kim EK, Kim MJ, Park YN, Park HJ, et al. Histogram analysis of gadoxetic acid-enhanced MRI for quantitative hepatic fibrosis measurement. PLoS One. 2014; 9:e114224. PMID: 25460180.
Article16. Lagadec M, Doblas S, Giraudeau C, Ronot M, Lambert SA, Fasseu M, et al. Advanced fibrosis: correlation between pharmacokinetic parameters at dynamic gadoxetate-enhanced MR imaging and hepatocyte organic anion transporter expression in rat liver. Radiology. 2015; 274:379–386. PMID: 25289480.
Article17. Dong D, Yin L, Qi Y, Xu L, Peng J. Protective effect of the total saponins from Rosa laevigata michx fruit against carbon tetrachloride-induced liver fibrosis in rats. Nutrients. 2015; 7:4829–4850. PMID: 26083117.
Article18. Tamada T, Prabhu V, Li J, Babb JS, Taneja SS, Rosenkrantz AB. Prostate cancer: diffusion-weighted MR imaging for detection and assessment of aggressiveness-comparison between conventional and kurtosis models. Radiology. 2017; 284:100–108. PMID: 28394755.19. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology. 1996; 24:289–293. PMID: 8690394.
Article20. Karlik SJ. Exploring and summarizing radiologic data. AJR Am J Roentgenol. 2003; 180:47–54. PMID: 12490475.
Article21. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988; 44:837–845. PMID: 3203132.
Article22. Liang HY, Huang YQ, Yang ZX, Ying D, Zeng MS, Rao SX. Potential of MR histogram analyses for prediction of response to chemotherapy in patients with colorectal hepatic metastases. Eur Radiol. 2016; 26:2009–2018. PMID: 26494642.
Article23. Taouli B, Chouli M, Martin AJ, Qayyum A, Coakley FV, Vilgrain V. Chronic hepatitis: role of diffusion-weighted imaging and diffusion-tensor imaging for the diagnosis of liver fibrosis and inflammation. J Magn Reson Imaging. 2008; 28:89–95. PMID: 18581382.24. Lambregts DM, Martens MH, Quah RC, Nikiforaki K, Heijnen LA, Dejong CH, et al. Whole-liver diffusion-weighted MRI histogram analysis: effect of the presence of colorectal hepatic metastases on the remaining liver parenchyma. Eur J Gastroenterol Hepatol. 2015; 27:399–404. PMID: 25874512.25. Anderson SW, Barry B, Soto J, Ozonoff A, O'Brien M, Jara H. Characterizing non-gaussian, high b-value diffusion in liver fibrosis: stretched exponential and diffusional kurtosis modeling. J Magn Reson Imaging. 2014; 39:827–834. PMID: 24259401.
Article26. Drevelegas K, Nikiforaki K, Constantinides M, Papanikolaou N, Papalavrentios L, Stoikou I, et al. Apparent diffusion coefficient quantification in determining the histological diagnosis of malignant liver lesions. J Cancer. 2016; 7:730–735. PMID: 27076855.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- RE: Diffusion-Weighted Imaging of Prostate Cancer: How Can We Use It Accurately?
- Utility of Readout-Segmented Echo-Planar Imaging-Based Diffusion Kurtosis Imaging for Differentiating Malignant from Benign Masses in Head and Neck Region
- Theoretical Background of Diffusion MR Imaging
- Advanced Magnetic Resonance Imaging for Pediatric Brain Tumors: Current Imaging Techniques and Interpretation Algorithms
- Quantitative Imaging in Pediatric Hepatobiliary Disease