Cancer Res Treat.
2015 Oct;47(4):607-615. 10.4143/crt.2014.249.
Phase I Study of OPB-31121, an Oral STAT3 Inhibitor, in Patients with Advanced Solid Tumors
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea. bangyj@snu.ac.kr
- 2Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 3Center for Gastric Cancer, National Cancer Center, Goyang, Korea.
- 4Department of Clinical Research and Development, Otsuka Pharmaceutical, Tokyo, Japan.
- 5Fujii Memorial Research Institute, Otsuka Pharmaceutical, Tokyo, Japan.
- KMID: 2403378
- DOI: http://doi.org/10.4143/crt.2014.249
Abstract
- PURPOSE
OPB-31121 is an oral STAT3 inhibitor with a good preclinical antitumor activity. This phase I dose-escalation study of OPB-31121 was conducted to determine maximum-tolerated dose (MTD), safety, pharmacokinetics, and preliminary antitumor efficacy in patients with advanced solid tumors.
MATERIALS AND METHODS
Patients received OPB-31121 once daily for 28 days of each cycle followed by 2 weeks rest. A standard 3+3 design was used for dose-escalation. Safety and response were evaluated by the National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) ver. 3.0 and Response Evaluation Criteria in Solid Tumor (RECIST) ver. 1.0, respectively.
RESULTS
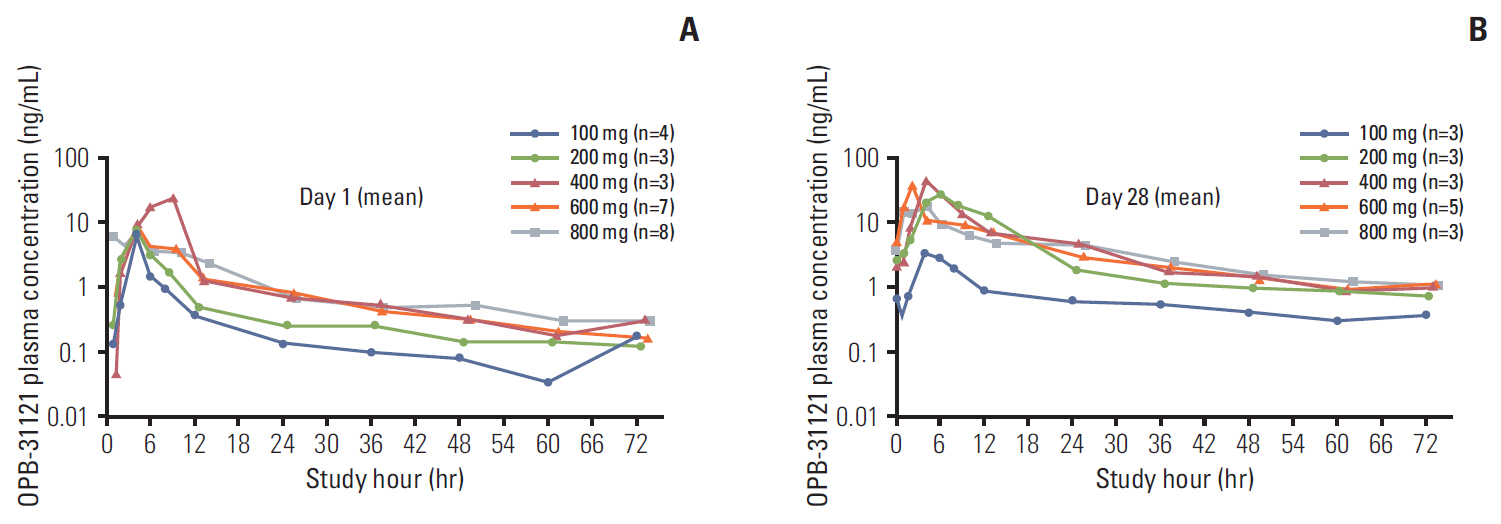
Twenty-five patients were treated with OPB-31121 at five dose levels: 100 mg (n=4), 200 mg (n=3), 400 mg (n=3), 600 mg (n=7), and 800 mg (n=8). Seven patients discontinued treatment during cycle 1 for various reasons other than study drug-related adverse events. Among 18 patients who were evaluable for dose-limiting toxicity (DLT), three DLTs were observed: one DLT (grade 3 vomiting) at 600 mg and two DLTs (grade 3 vomiting, grade 3 diarrhea) at 800 mg. The MTD was determined as 800 mg/day. Common adverse events were gastrointestinal adverse event including nausea (84%), vomiting (80%), and diarrhea (72%). Pharmacokinetics did not demonstrate dose-proportionality of OPB-31121. Eight patients had stable disease and 10 patients had disease progression. Two patients (1 colon cancer, 1 rectal cancer) showed tumor shrinkage. One gastric cancer patient continued treatment up to cycle 13 before disease progression.
CONCLUSION
This study demonstrates feasibility of STAT3 inhibition in patients with advanced solid tumor. OPB-31121, at the MTD of 800 mg/day, was safe and relatively well tolerated, and has a preliminary antitumor activity.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Phase I Dose-Finding Study of OPB-111077, a Novel STAT3 Inhibitor, in Patients with Advanced Hepatocellular Carcinoma
Changhoon Yoo, Jihoon Kang, Ho Yeong Lim, Jee Hyun Kim, Myung-Ah Lee, Kyung-Hun Lee, Tae-You Kim, Baek-Yeol Ryoo
Cancer Res Treat. 2019;51(2):510-518. doi: 10.4143/crt.2018.226.
Reference
-
References
1. Yu H, Jove R. The STATs of cancer: new molecular targets come of age. Nat Rev Cancer. 2004; 4:97–105.2. Mankan AK, Greten FR. Inhibiting signal transducer and activator of transcription 3: rationality and rationale design of inhibitors. Expert Opin Investig Drugs. 2011; 20:1263–75.
Article3. Sansone P, Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol. 2012; 30:1005–14.
Article4. Calvisi DF, Ladu S, Gorden A, Farina M, Conner EA, Lee JS, et al. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology. 2006; 130:1117–28.
Article5. Jove R. Preface: STAT signaling. Oncogene. 2000; 19:2466–7.
Article6. Levy DE, Inghirami G. STAT3: a multifaceted oncogene. Proc Natl Acad Sci U S A. 2006; 103:10151–2.
Article7. Yu H, Pardol D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009; 9:798–809.
Article8. Alvarez JV, Greulich H, Sellers WR, Meyerson M, Frank DA. Signal transducer and activator of transcription 3 is required for the oncogenic effects of non-small-cell lung cancer-associated mutations of the epidermal growth factor receptor. Cancer Res. 2006; 66:3162–8.
Article9. Grandis JR, Drenning SD, Zeng Q, Watkins SC, Melhem MF, Endo S, et al. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc Natl Acad Sci U S A. 2000; 97:4227–32.
Article10. Mizutani Y, Bonavida B, Koishihara Y, Akamatsu K, Ohsugi Y, Yoshida O. Sensitization of human renal cell carcinoma cells to cis-diamminedichloroplatinum(II) by anti-interleukin 6 monoclonal antibody or anti-interleukin 6 receptor monoclonal antibody. Cancer Res. 1995; 55:590–6.11. Alas S, Bonavida B. Inhibition of constitutive STAT3 activity sensitizes resistant non-Hodgkin's lymphoma and multiple myeloma to chemotherapeutic drug-mediated apoptosis. Clin Cancer Res. 2003; 9:316–26.12. Li WC, Ye SL, Sun RX, Liu YK, Tang ZY, Kim Y, et al. Inhibition of growth and metastasis of human hepatocellular carcinoma by antisense oligonucleotide targeting signal transducer and activator of transcription 3. Clin Cancer Res. 2006; 12:7140–8.
Article13. Verstovsek S. Therapeutic potential of JAK2 inhibitors. Hematology Am Soc Hematol Educ Program. 2009. 636–42.
Article14. Kim MJ, Nam HJ, Kim HP, Han SW, Im SA, Kim TY, et al. OPB-31121, a novel small molecular inhibitor, disrupts the JAK2/STAT3 pathway and exhibits an antitumor activity in gastric cancer cells. Cancer Lett. 2013; 335:145–52.
Article15. Bendell JC, Hong DS, Burris HA 3rd, Naing A, Jones SF, Falchook G, et al. Phase 1, open-label, dose-escalation, and pharmacokinetic study of STAT3 inhibitor OPB-31121 in subjects with advanced solid tumors. Cancer Chemother Pharmacol. 2014; 74:125–30.
Article16. Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005; 7:387–97.
Article17. Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005; 365:1054–61.
Article18. Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005; 352:1779–90.19. James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005; 434:1144–8.
Article20. Lin L, Liu A, Peng Z, Lin HJ, Li PK, Li C, et al. STAT3 is necessary for proliferation and survival in colon cancer-initiating cells. Cancer Res. 2011; 71:7226–37.
Article21. Lin L, Liu A, Peng Z, Lin HJ, Li PK, Li C, et al. STAT3 is necessary for proliferation and survival in colon cancer-initiating cells. Cancer Res. 2011; 71:7226–37.
Article22. Morikawa T, Baba Y, Yamauchi M, Kuchiba A, Nosho K, Shima K, et al. STAT3 expression, molecular features, inflammation patterns, and prognosis in a database of 724 colorectal cancers. Clin Cancer Res. 2011; 17:1452–62.
Article23. Chen J, Wang J, Lin L, He L, Wu Y, Zhang L, et al. Inhibition of STAT3 signaling pathway by nitidine chloride suppressed the angiogenesis and growth of human gastric cancer. Mol Cancer Ther. 2012; 11:277–87.
Article24. Deng JY, Sun D, Liu XY, Pan Y, Liang H. STAT-3 correlates with lymph node metastasis and cell survival in gastric cancer. World J Gastroenterol. 2010; 16:5380–7.
Article25. Gong W, Wang L, Yao JC, Ajani JA, Wei D, Aldape KD, et al. Expression of activated signal transducer and activator of transcription 3 predicts expression of vascular endothelial growth factor in and angiogenic phenotype of human gastric cancer. Clin Cancer Res. 2005; 11:1386–93.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Phase I Dose-Finding Study of OPB-111077, a Novel STAT3 Inhibitor, in Patients with Advanced Hepatocellular Carcinoma
- Phase 1/2a Study of Rivoceranib, a Selective VEGFR-2 Angiogenesis Inhibitor, in Patients with Advanced Solid Tumors
- Effect of STAT3 on Lysophosphatidic Acid-Induced Oral Cancer Cell Invasion
- Immunotherapy in Pediatric Solid Tumors
- The Expression of STAT3 in Skin Tumors