Intest Res.
2018 Jan;16(1):116-125. 10.5217/ir.2018.16.1.116.
Topographic, histological and molecular study of aberrant crypt foci identified in human colon in different clinical groups
- Affiliations
-
- 1Department of Pathology, All India Institute of Medical Sciences, New Delhi, India. prasenaiims@gmail.com
- 2Department of Biostatistics, All India Institute of Medical Sciences, New Delhi, India.
- 3Department of Gastrointestinal Surgery, All India Institute of Medical Sciences, New Delhi, India.
- KMID: 2402655
- DOI: http://doi.org/10.5217/ir.2018.16.1.116
Abstract
- BACKGROUND/AIMS
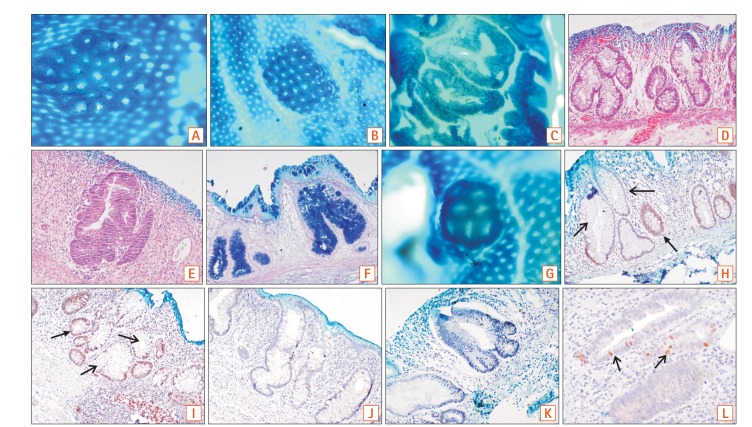
Aberrant crypt foci (ACF) are early microscopic lesions of the colonic mucosa, which can be detected by magnified chromoendoscopy. Herein, we have investigated whether ACF identified in different clinical groups can be differentiated based on their characteristics.
METHODS
Macroscopically unremarkable mucosal flaps were collected from 270 fresh colectomies and divided into 3 clinical groups: colorectal carcinoma (group A), disease controls having known pre-neoplastic potential (group Bc), and disease controls without risk of carcinoma development (group Bn). Topographic and histologic analysis, immunohistochemistry, and molecular studies (high-resolution melt curve analysis, real-time polymerase chain reaction, and Sanger sequencing) were conducted for certain neoplasia-associated markers.
RESULTS
ACF were seen in 107 cases, out of which 72 were left colonic ACF and 35 right colonic ACF (67.2% vs. 32.7%, P=0.02). The overall density of left colonic ACF was 0.97/cm, which was greater than the right colonic ACF density of 0.81/cm. Hypercrinia was present in 41 out of 72 left colonic ACF and in 14 out of 35 right colonic ACF (P=0.01). Immunohistochemical expression of p53 was also greater in left colonic ACF than in right colonic ACF (60.5% vs. 38.2%, P=0.03). However, ACF identified among the 3 clinical groups did not show any distinguishing topographic, histological, or genetic changes.
CONCLUSIONS
Left colonic ACF appear to be high-risk based on their morphological and prototypic tumor marker signature. ACF identified in different clinical groups do not show significant genotypic or topographic differences. Further detailed genetic studies are required to elucidate them further.
Keyword
MeSH Terms
Figure
Reference
-
1. Pathy S, Lambert R, Sauvaget C, Sankaranarayanan R. The incidence and survival rates of colorectal cancer in India remain low compared with rising rates in East Asia. Dis Colon Rectum. 2012; 55:900–906. PMID: 22810477.
Article2. Indian Council of Medical Research. ICMR Bulletin. New Delhi, India: Indian Council of Medical Research;2010.3. Mohandas KM. Colorectal cancer in India: controversies, enigmas and primary prevention. Indian J Gastroenterol. 2011; 30:3–6. PMID: 21222189.
Article4. Luo Y, Yu M, Grady WM. Field cancerization in the colon: a role for aberrant DNA methylation? Gastroenterol Rep (Oxf). 2014; 2:16–20. PMID: 24760232.
Article5. Bird RP. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett. 1987; 37:147–151. PMID: 3677050.
Article6. Caderni G, Femia AP, Giannini A, et al. Identification of mucin-depleted foci in the unsectioned colon of azoxymethane-treated rats: correlation with carcinogenesis. Cancer Res. 2003; 63:2388–2392. PMID: 12750256.7. Takayama T, Ohi M, Hayashi T, et al. Analysis of K-ras, APC, and beta-catenin in aberrant crypt foci in sporadic adenoma, cancer, and familial adenomatous polyposis. Gastroenterology. 2001; 121:599–611. PMID: 11522744.
Article8. Inoue A, Okamoto K, Fujino Y, et al. B-RAF mutation and accumulated gene methylation in aberrant crypt foci (ACF), sessile serrated adenoma/polyp (SSA/P) and cancer in SSA/P. Br J Cancer. 2015; 112:403–412. PMID: 25314065.
Article9. Gupta B, Das P, Ghosh S, et al. Identification of high-risk aberrant crypt foci and mucin-depleted foci in the human colon with study of colon cancer stem cell markers. Clin Colorectal Cancer. 2017; 16:204–213. PMID: 27789195.
Article10. Kudo S, Hirota S, Nakajima T, et al. Colorectal tumours and pit pattern. J Clin Pathol. 1994; 47:880–885. PMID: 7962600.
Article11. Das P, Jain D, Vaiphei K, Wig JD. Abberant crypt foci–importance in colorectal carcinogenesis and expression of p53 and mdm2: a changing concept. Dig Dis Sci. 2008; 53:2183–2188. PMID: 18080767.
Article12. Benlloch S, Payá A, Alenda C, et al. Detection of BRAF V600E mutation in colorectal cancer: comparison of automatic sequencing and real-time chemistry methodology. J Mol Diagn. 2006; 8:540–543. PMID: 17065421.13. Sakai E, Takahashi H, Kato S, et al. Investigation of the prevalence and number of aberrant crypt foci associated with human colorectal neoplasm. Cancer Epidemiol Biomarkers Prev. 2011; 20:1918–1924. PMID: 21750169.
Article14. Roncucci L, Pedroni M, Vaccina F, Benatti P, Marzona L, De Pol A. Aberrant crypt foci in colorectal carcinogenesis: cell and crypt dynamics. Cell Prolif. 2000; 33:1–18. PMID: 10741640.
Article15. Roncucci L, Medline A, Bruce WR. Classification of aberrant crypt foci and microadenomas in human colon. Cancer Epidemiol Biomarkers Prev. 1991; 1:57–60. PMID: 1845171.16. Cheng L, Lai MD. Aberrant crypt foci as microscopic precursors of colorectal cancer. World J Gastroenterol. 2003; 9:2642–2649. PMID: 14669304.
Article17. de Jong AE, van Puijenbroek M, Hendriks Y, et al. Microsatellite instability, immunohistochemistry, and additional PMS2 staining in suspected hereditary nonpolyposis colorectal cancer. Clin Cancer Res. 2004; 10:972–980. PMID: 14871975.
Article18. Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry. J Mol Diagn. 2008; 10:293–300. PMID: 18556767.
Article19. Kim H, Jen J, Vogelstein B, Hamilton SR. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol. 1994; 145:148–156. PMID: 8030745.20. Young J, Simms LA, Biden KG, et al. Features of colorectal cancers with high-level microsatellite instability occurring in familial and sporadic settings: parallel pathways of tumorigenesis. Am J Pathol. 2001; 159:2107–2116. PMID: 11733361.
Article21. Deng G, Chen A, Hong J, Chae HS, Kim YS. Methylation of CpG in a small region of the hMLH1 promoter invariably correlates with the absence of gene expression. Cancer Res. 1999; 59:2029–2033. PMID: 10232580.22. Giovannucci E, Ogino S. DNA methylation, field effects, and colorectal cancer. J Natl Cancer Inst. 2005; 97:1317–1319. PMID: 16174847.
Article23. Jass JR, Whitehall VL, Young J, Leggett BA. Emerging concepts in colorectal neoplasia. Gastroenterology. 2002; 123:862–876. PMID: 12198712.
Article24. Greenspan EJ, Cyr JL, Pleau DC, et al. Microsatellite instability in aberrant crypt foci from patients without concurrent colon cancer. Carcinogenesis. 2007; 28:769–776. PMID: 17088260.
Article25. Yamashita N, Minamoto T, Ochiai A, Onda M, Esumi H. Frequent and characteristic K-ras activation and absence of p53 protein accumulation in aberrant crypt foci of the colon. Gastroenterology. 1995; 108:434–440. PMID: 7835585.
Article26. Rosenberg DW, Yang S, Pleau DC, et al. Mutations in BRAF and KRAS differentially distinguish serrated versus non-serrated hyperplastic aberrant crypt foci in humans. Cancer Res. 2007; 67:3551–3554. PMID: 17440063.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Aberrant Crypt Foci: Histopathologic Classification and Profiles of Mucin Secretion
- Aberrant Crypt Foci in the Background Mucosa of Colorectal Adenocarcinoma
- Dose-response assessment of the anti-cancer efficacy of soy isoflavones in dimethylhydrazine-treated rats fed 6% fructooligosaccharide
- Sequential Changes in Aberrant Crypt Foci and Lectin Expression in the Early and Late Stages of DMH-Induced Colon Carcinogenesis in Rats
- Suppressive Effect of Zinc on the Formation of Colonic Preneoplastic Lesions in the Mouse Fed High Levels of Dietary Iron