Intest Res.
2018 Jan;16(1):99-108. 10.5217/ir.2018.16.1.99.
An analysis of dietary fiber and fecal fiber components including pH in rural Africans with colorectal cancer
- Affiliations
-
- 1Department of Pathology, Ahmadu Bello University Faculty of Medicine, Zaria, Nigeria. fmohammed@abu.edu.ng
- 2Department of Pathology, Ahmadu Bello University Teaching Hospital, Zaria, Nigeria.
- 3Department of Biochemistry, Ahmadu Bello University Faculty of Sciences, Zaria, Nigeria.
- 4Department of Surgery, Ahmadu Bello University Faculty of Medicine, Zaria, Nigeria.
- 5Department of Surgery Zaria, Ahmadu Bello University Teaching Hospital, Zaria, Nigeria.
- 6Department of Radiotherapy, Ahmadu Bello University Faculty of Medicine, Zaria, Nigeria.
- 7Department of Radiotherapy, Ahmadu Bello University Teaching Hospital, Zaria, Nigeria.
- 8Department of Haematology and Blood Transfusion, Ahmadu Bello University Faculty of Medicine, Zaria, Nigeria.
- 9Department of Haematology and Blood Transfusion, Ahmadu Bello University Teaching Hospital, Shika Zaria, Nigeria.
- 10Department of Radiation Oncology, University of Ibadan, Ibadan, Nigeria.
- 11Department of Radiation Oncology, University College Hospital, Ibadan, Nigeria.
- 12Department of Biomedical Science, University of Wolverhampton, Wolverhampton, UK.
- KMID: 2402653
- DOI: http://doi.org/10.5217/ir.2018.16.1.99
Abstract
- BACKGROUND/AIMS
Colorectal cancer (CRC) is now a major public health problem with heavy morbidity and mortality in rural Africans despite the lingering dietary fiber-rich foodstuffs consumption. Studies have shown that increased intake of dietary fiber which contribute to low fecal pH and also influences the activity of intestinal microbiota, is associated with a lowered risk for CRC. However, whether or not the apparent high dietary fiber consumption by Africans do not longer protects against CRC risk is unknown. This study evaluated dietary fiber intake, fecal fiber components and pH levels in CRC patients.
METHODS
Thirty-five subjects (CRC=21, control=14), mean age 45 years were recruited for the study. A truncated food frequency questionnaire and modified Goering and Van Soest procedures were used.
RESULTS
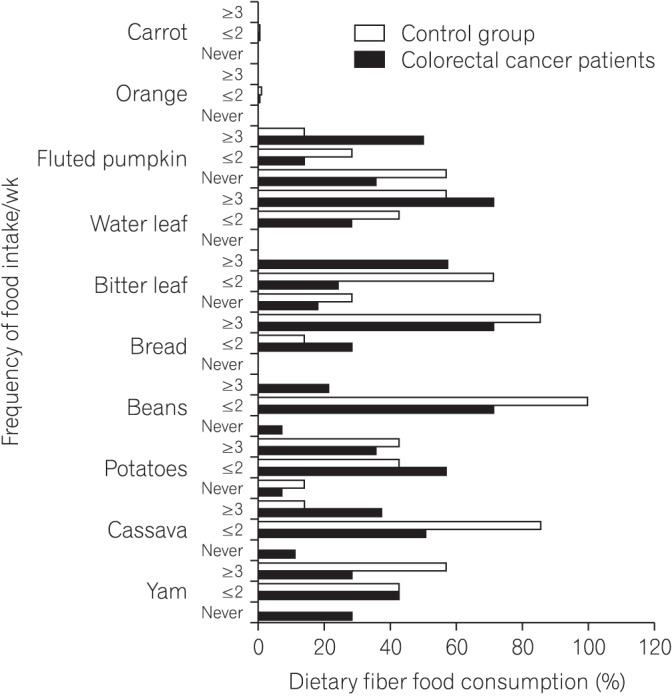
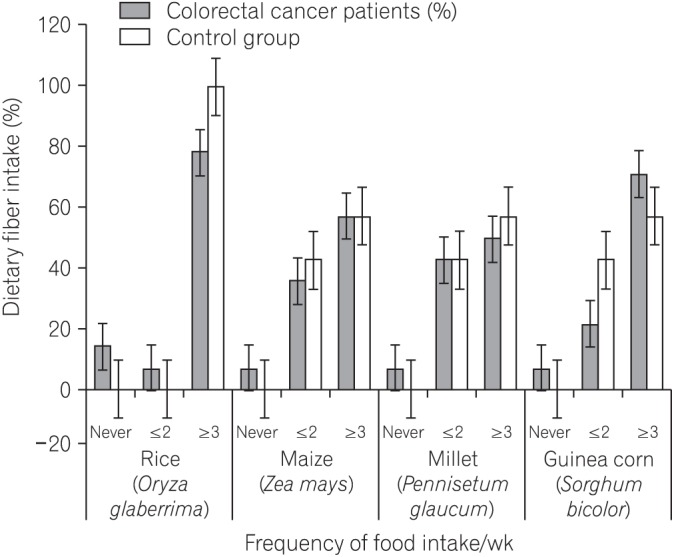
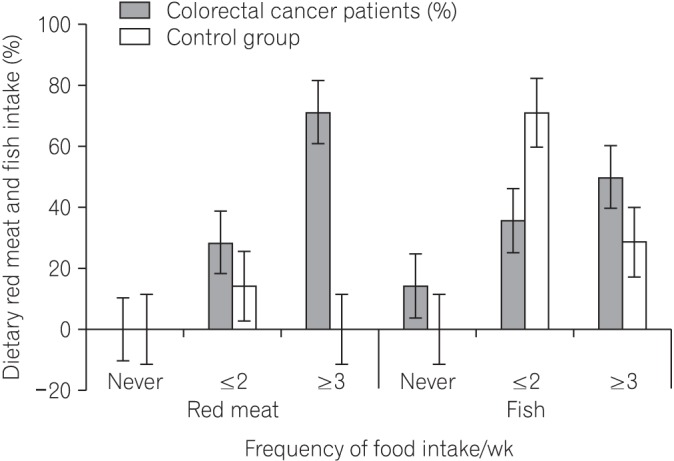
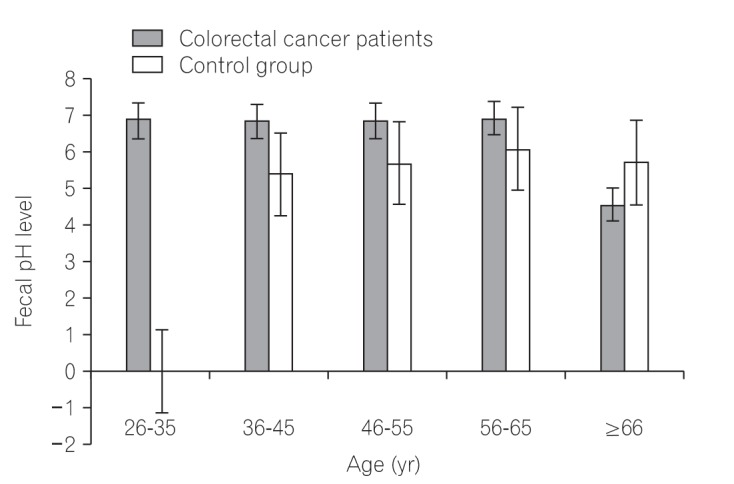
We found that all subjects consumed variety of dietary fiber-rich foodstuffs. There is slight preponderance in consumption of dietary fiber by the control group than the CRC patients. We also found a significant difference in the mean fecal neutral detergent fiber, acid detergent fiber, hemicellulose, cellulose and lignin contents from the CRC patients compared to the controls (P < 0.05). The CRC patients had significantly more fecal pH level than the matched apparently healthy controls (P=0.017).
CONCLUSIONS
The identified differences in the fecal fiber components and stool pH levels between the 2 groups may relate to CRC incidence and mortality in rural Africans. There is crucial need for more hypothesis-driven research with adequate funding on the cumulative preventive role of dietary fiber-rich foodstuffs against colorectal cancer in rural Africans "today."
Keyword
MeSH Terms
Figure
Reference
-
1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014; 64:9–29. PMID: 24399786.
Article2. Katsidzira L, Gangaidzo I, Thomson S, Rusakaniko S, Matenga J, Ramesar R. The shifting epidemiology of colorectal cancer in sub-Saharan Africa. Lancet Gastroenterol Hepatol. 2017; 2:377–383. PMID: 28397702.
Article3. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–E386. PMID: 25220842.
Article4. Irabor DO. Colorectal carcinoma in West Africans: some considerations on its relatively lower incidence compared to caucasians. Pak J Med Sci. 2008; 24:331–335.5. O'Keefe SJ, Li JV, Lahti L, et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun. 2015; 6:6342. DOI: 10.1038/ncomms7342. PMID: 25919227.6. Tuan J, Chen YX. Dietary and lifestyle factors associated with colorectal cancer risk and interactions with microbiota: fiber, red or processed meat and alcoholic drinks. Gastrointest Tumors. 2016; 3:17–24. PMID: 27722153.
Article7. Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014; 12:661–672. PMID: 25198138.
Article8. Dziedzic K, Szwengiel A, Górecka D, et al. Effect of wheat dietary fiber particle size during digestion in vitro on bile acid, faecal bacteria and short-chain fatty acid content. Plant Foods Hum Nutr. 2016; 71:151–157. PMID: 26924312.
Article9. Drasar BS, Jenkins DJ. Bacteria, diet, and large bowel cancer. Am J Clin Nutr. 1976; 29:1410–1416. PMID: 998551.
Article10. Sugawara M, Suzuki K, Endo K, Kumemura M, Takeuchi M, Mitsuoka T. Effect of the dietary supplementation of corn hemicellulose on fecal flora and bacterial enzyme activities in human adults. Agric Biol Chem. 1990; 54:1683–1688.
Article11. Nagao-Kitamoto H, Kitamoto S, Kuffa P, Kamada N. Pathogenic role of the gut microbiota in gastrointestinal diseases. Intest Res. 2016; 14:127–138. PMID: 27175113.
Article12. Goering HK, Van Soest PJ. Forage fiber analyses: apparatus, reagents, procedures, and some applications. Washington DC: U.S. Agricultural Research Service;1970.13. Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991; 74:3583–3597. PMID: 1660498.
Article14. Iliyasu Y, Ladipo JK, Akang EE, Adebamowo CA, Ajao OG, Aghadiuno PU. A twenty-year review of malignant colorectal neoplasms at University College Hospital, Ibadan, Nigeria. Dis Colon Rectum. 1996; 39:536–540. PMID: 8620804.
Article15. Leufkens AM, Van Duijnhoven FJ, Boshuizen HC, et al. Educational level and risk of colorectal cancer in EPIC with specific reference to tumor location. Int J Cancer. 2012; 130:622–630. PMID: 21412763.
Article16. Uchida K, Kono S, Yin G, et al. Dietary fiber, source foods and colorectal cancer risk: the Fukuoka Colorectal Cancer Study. Scand J Gastroenterol. 2010; 45:1223–1231. PMID: 20500015.
Article17. Cheah PY, Bernstein H. Colon cancer and dietary fiber: cellulose inhibits the DNA-damaging ability of bile acids. Nutr Cancer. 1990; 13:51–57. PMID: 2153952.
Article18. Abe SK, Inoue M, Sawada N, et al. Rice, bread, noodle and cereal intake and colorectal cancer in Japanese men and women: the Japan Public Health Center-based prospective Study (JPHC Study). Br J Cancer. 2014; 110:1316–1321. PMID: 24384682.
Article19. Pérez-Jiménez J, Díaz-Rubio ME, Mesías M, Morales FJ, Saura-Calixto F. Evidence for the formation of maillardized insoluble dietary fiber in bread: a specific kind of dietary fiber in thermally processed food. Food Res Int. 2014; 55:391–396.
Article20. Skeie G, Braaten T, Olsen A, et al. Whole grain intake and survival among Scandinavian colorectal cancer patients. Nutr Cancer. 2014; 66:6–13. PMID: 24274588.
Article21. O'Callaghan NJ, Toden S, Bird AR, Topping DL, Fenech M, Conlon MA. Colonocyte telomere shortening is greater with dietary red meat than white meat and is attenuated by resistant starch. Clin Nutr. 2012; 31:60–64. PMID: 21963168.22. Hall MN, Chavarro JE, Lee IM, Willett WC, Ma J. A 22-year prospective study of fish, n-3 fatty acid intake, and colorectal cancer risk in men. Cancer Epidemiol Biomarkers Prev. 2008; 17:1136–1143. PMID: 18483335.
Article23. Eltweri AM, Thomas AL, Metcalfe M, Calder PC, Dennison AR, Bowrey DJ. Potential applications of fish oils rich in omega-3 polyunsaturated fatty acids in the management of gastrointestinal cancer. Clin Nutr. 2017; 36:65–78. PMID: 26833289.
Article24. Rodríguez-Romero N, Abecia L, Fondevila M, et al. Effects of levels of insoluble and soluble fibre in diets for growing rabbits on faecal digestibility, nitrogen recycling and in vitro fermentation. World Rabbit Sci. 2011; 19:85–94.
Article25. Singh A, Singh SN. Dietary fiber content of Indian diets. Asian J Pharm Clin Res. 2015; 8:58–61.26. Zhang YJ, Li S, Gan RY, Zhou T, Xu DP, Li HB. Impacts of gut bacteria on human health and diseases. Int J Mol Sci. 2015; 16:7493–7519. PMID: 25849657.
Article27. Biddle A, Stewart L, Blanchard J, Leschine S. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity. 2013; 5:627–640.
Article28. Marchesi JR, Dutilh BE, Hall N, et al. Towards the human colorectal cancer microbiome. PLoS One. 2011; 6:e20447. DOI: 10.1371/journal.pone.0020447. PMID: 21647227.
Article29. Salyers AA, Balascio RJ, Palmer JK. Breakdown of xylan by enzymes from human colonic bacteria. J Food Biochem. 1982; 6:39–55.
Article30. Sloan DA, Fleiszer DM, Richards GK, Murray D, Brown RA. The effect of the fiber components cellulose and lignin on experimental colon neoplasia. J Surg Oncol. 1993; 52:77–82. PMID: 8385723.
Article31. Degirolamo C, Modica S, Palasciano G, Moschetta A. Bile acids and colon cancer: solving the puzzle with nuclear receptors. Trends Mol Med. 2011; 17:564–572. PMID: 21724466.
Article32. Ajouz H, Mukherji D, Shamseddine A. Secondary bile acids: an underrecognized cause of colon cancer. World J Surg Oncol. 2014; 12:164. PMID: 24884764.33. Lee S, Monnappa AK, Mitchell RJ. Biological activities of lignin hydrolysate-related compounds. BMB Rep. 2012; 45:265–274. PMID: 22617449.34. Ferguson LR, Harris PJ. Studies on the role of specific dietary fibres in protection against colorectal cancer. Mutat Res. 1996; 350:173–184. PMID: 8657179.35. Hester CM, Jala VR, Langille MG, Umar S, Greiner KA, Haribabu B. Fecal microbes, short chain fatty acids, and colorectal cancer across racial/ethnic groups. World J Gastroenterol. 2015; 21:2759–2769. PMID: 25759547.36. Ohigashi S, Sudo K, Kobayashi D, et al. Changes of the intestinal microbiota, short chain fatty acids, and fecal pH in patients with colorectal cancer. Dig Dis Sci. 2013; 58:1717–1726. PMID: 23306850.37. Donohoe DR, Holley D, Collins LB, et al. A gnotobiotic mouse model demonstrates that dietary fiber protects against colorectal tumorigenesis in a microbiota- and butyrate-dependent manner. Cancer Discov. 2014; 4:1387–1397. PMID: 25266735.
Article38. Bordonaro M, Lazarova DL, Sartorelli AC. Butyrate and Wnt signaling: a possible solution to the puzzle of dietary fiber and colon cancer risk? Cell Cycle. 2008; 7:1178–1183. PMID: 18418037.
Article39. Bultman SJ. Molecular pathways: gene-environment interactions regulating dietary fiber induction of proliferation and apoptosis via butyrate for cancer prevention. Clin Cancer Res. 2014; 20:799–803. PMID: 24270685.
Article40. de Kok TM, van Faassen A, Glinghammar B, et al. Bile acid concentrations, cytotoxicity, and pH of fecal water from patients with colorectal adenomas. Dig Dis Sci. 1999; 44:2218–2225. PMID: 10573365.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Estimation of Nutrients Intakes and Dietary Fiber Intake of Teenagers in Urban, Fishing, and Rural Areas.
- Analysis of the Relationship between Dietary Fiber Intake & Food Habits in the Korean Adult Population: Using the 2001 Korean National Health and Nutrition Survey Data and the Newly Established Dietary Fiber Database
- Effect of Physical Activity on the Association Between Dietary Fiber and Constipation: Evidence From the National Health and Nutrition Examination Survey 2005-2010
- Analysis of dietary insoluble and soluble fiber contents in school meal
- Association Between Colon Diverticulosis and Development of Colorectal Cancer