Anat Cell Biol.
2017 Dec;50(4):247-254. 10.5115/acb.2017.50.4.247.
Morphological classification and comparison of suboccipital muscle fiber characteristics
- Affiliations
-
- 1Department of Anatomy, Tokyo Dental College, Tokyo, Japan. myamauchi@tdc.ac.jp
- 2Department of Histology and Developmental Biology, Tokyo Dental College, Tokyo, Japan.
- KMID: 2399892
- DOI: http://doi.org/10.5115/acb.2017.50.4.247
Abstract
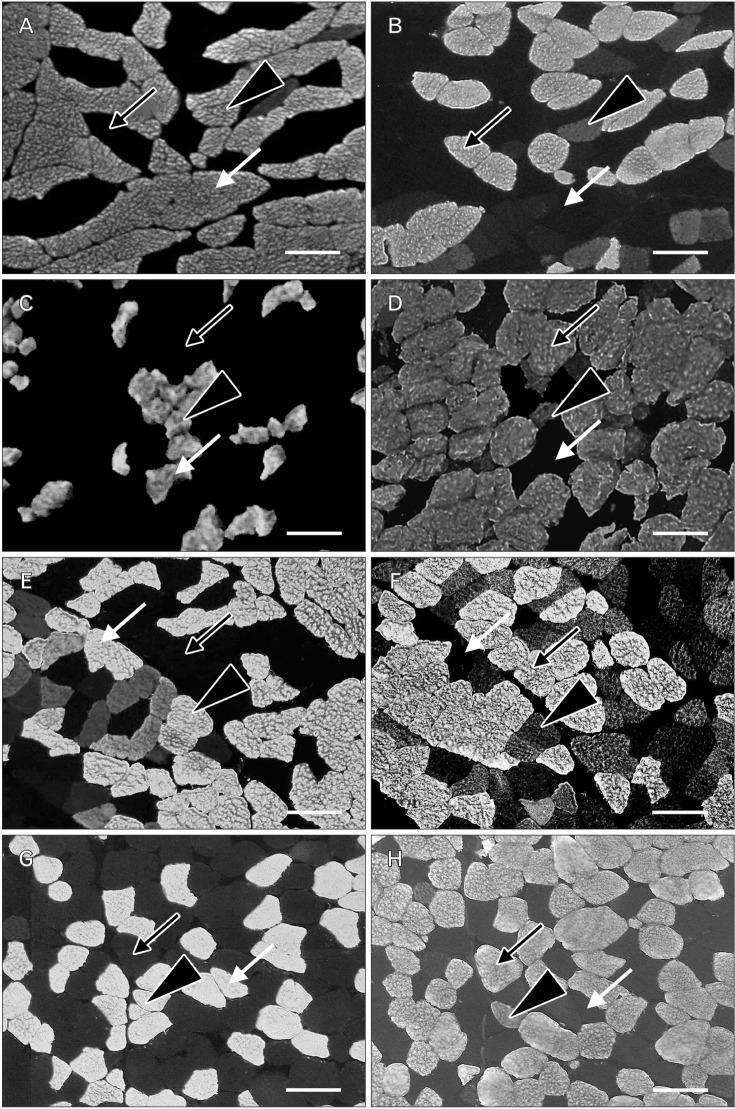
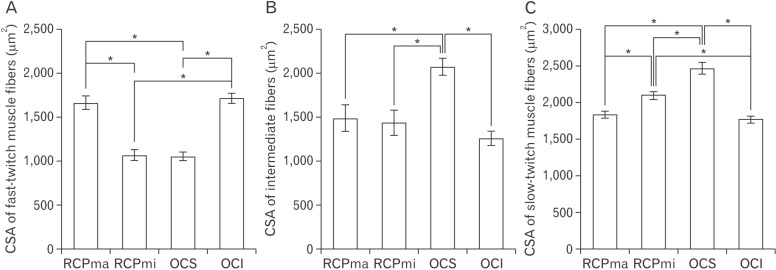
- In an attempt to clarify the function of the suboccipital muscles, we performed morphological observation of the suboccipital muscles for variations in the muscle belly and compared the morphology of their muscle fibers in terms of cross-sectional area by immunostaining with anti-myosin heavy chain antibodies. The cadavers of 25 Japanese individuals were used: 22 for morphological examinations and three for histological examinations. Among samples of the rectus capitis posterior major muscle (RCPma) and rectus capitis posterior minor muscle (RCPmi), 86.4% had a typical muscle appearance with a single belly, and 13.6% had an anomalous morphology. None of the samples of the obliquus capitis superior (OCS) or obliquus capitis inferior (OCI) muscles had an anomalous appearance. Measurement of cross-sectional area revealed that fast-twitch muscle fibers in the RCPma and OCI had a significantly greater cross-sectional area than those of the RCPmi and OCS. The cross-sectional area of intermediate muscle fibers was also significantly greater in the OCS than in the RCPma, RCPmi, and OCI. The cross-sectional area of slow-twitch muscle fibers was significantly greater in the OCS than in the RCPma, RCPmi, and OCI, and the RCPmi showed a significantly greater cross-sectional area for slow-twitch muscle fibers than did the RCPma, and OCI. Our findings indicate that the RCPmi and OCS exert a greater force than the RCPma and OCI, and act as anti-gravity agonist muscles of the head. Prolonged head extension in individuals with anomalous suboccipital muscle groups could result in dysfunction due to undue stress.
MeSH Terms
Figure
Cited by 1 articles
-
An anatomical investigation of the suboccipital- and inferior suboccipital triangles
Kirsten Shannon Regan, Gerda Venter
Anat Cell Biol. 2023;56(3):350-359. doi: 10.5115/acb.23.015.
Reference
-
1. Hiatt JL, Gartner LP. Textbook of head and neck anatomy. 2nd ed. Baltimore, MD: Williams & Wilkins;1987. p. 109–122.2. McPartland JM, Brodeur RR, Hallgren RC. Chronic neck pain, standing balance, and suboccipital muscle atrophy: a pilot study. J Manipulative Physiol Ther. 1997; 20:24–29. PMID: 9004119.3. Loukas M, Tubbs RS. An accessory muscle within the suboccipital triangle. Clin Anat. 2007; 20:962–963. PMID: 17415745.4. Tagil SM, Ozçakar L, Bozkurt MC. Insight into understanding the anatomical and clinical aspects of supernumerary rectus capitis posterior muscles. Clin Anat. 2005; 18:373–375. PMID: 15971221.5. Nayak SR, Swamy R, Krishnamurthy A, Dasgupta H. Bilateral anomaly of rectus capitis posterior muscles in the suboccipital triangle and its clinical implication. Clin Ter. 2011; 162:355–356. PMID: 21912824.6. Bergmann RA, Thompson SA, Afifi AK, Saadeh FA. Compendium of human anatomic variation. Baltimore, MD: Urban & Schwarzennberg;1988. p. 33–34.7. Mori M. Statistics on the Musculature of the Japanese. Okajimas Folia Anat Jpn. 1964; 40:195–300. PMID: 14213705.8. Abe S, Maejima M, Watanabe H, Shibahara T, Agematsu H, Doi T, Sakiyama K, Usami A, Gojyo K, Hashimoto M, Yoshinari M, Ide Y. Muscle-fiber characteristics in adult mouse-tongue muscles. Anat Sci Int. 2002; 77:145–148. PMID: 12418096.9. Doi T, Abe S, Ide Y. Masticatory function and properties of masseter muscle fibers in microphthalmic (mi/mi) mice during postnatal development. Ann Anat. 2003; 185:435–440. PMID: 14575270.10. Feng X, Zhang T, Xu Z, Choi SJ, Qian J, Furdui CM, Register TC, Delbono O. Myosin heavy chain isoform expression in the Vastus Lateralis muscle of aging African green vervet monkeys. Exp Gerontol. 2012; 47:601–607. PMID: 22617406.11. Pette D, Staron RS. Cellular and molecular diversities of mammalian skeletal muscle fibers. Rev Physiol Biochem Pharmacol. 1990; 116:1–76. PMID: 2149884.12. Henneman E, Olson CB. Relations between structure and function in the design of skeletal muscles. J Neurophysiol. 1965; 28:581–598. PMID: 14328455.13. Sartorius CA, Lu BD, Acakpo-Satchivi L, Jacobsen RP, Byrnes WC, Leinwand LA. Myosin heavy chains IIa and IId are functionally distinct in the mouse. J Cell Biol. 1998; 141:943–953. PMID: 9585413.14. Yanagisawa N, Abe S, Agematsu H, Sakiyama K, Usami A, Tamatsu Y, Ide Y. Myosin heavy chain composition of tongue muscle in microphthalmic (mi/mi) mice before and after weaning. Ann Anat. 2006; 188:329–336. PMID: 16856597.15. Chang H, Gilbertson LG, Goel VK, Winterbottom JM, Clark CR, Patwardhan A. Dynamic response of the occipito-atlanto-axial (C0-C1-C2) complex in right axial rotation. J Orthop Res. 1992; 10:446–453. PMID: 1569507.16. Johnson GM, Zhang M, Jones DG. The fine connective tissue architecture of the human ligamentum nuchae. Spine (Phila Pa 1976). 2000; 25:5–9. PMID: 10647153.17. Kulkarni V, Chandy MJ, Babu KS. Quantitative study of muscle spindles in suboccipital muscles of human foetuses. Neurol India. 2001; 49:355–359. PMID: 11799407.18. Kamibayashi LK, Richmond FJ. Morphometry of human neck muscles. Spine (Phila Pa 1976). 1998; 23:1314–1323. PMID: 9654620.19. Ikai M, Fukunaga T. Calculation of muscle strength per unit cross-sectional area of human muscle by means of ultrasonic measurement. Int Z Angew Physiol. 1968; 26:26–32. PMID: 5700894.20. Rantanen J, Rissanen A, Kalimo H. Lumbar muscle fiber size and type distribution in normal subjects. Eur Spine J. 1994; 3:331–335. PMID: 7532536.21. Katori Y, Yamamoto M, Asakawa S, Maki H, Rodríguez-Vázquez JF, Murakami G, Abe S. Fetal developmental change in topographical relationship between the human lateral pterygoid muscle and buccal nerve. J Anat. 2012; 220:384–395. PMID: 22352373.22. Alix ME, Bates DK. A proposed etiology of cervicogenic headache: the neurophysiologic basis and anatomic relationship between the dura mater and the rectus posterior capitis minor muscle. J Manipulative Physiol Ther. 1999; 22:534–539. PMID: 10543584.23. Fernández-de-Las-Peñas C, Cuadrado ML, Arendt-Nielsen L, Ge HY, Pareja JA. Association of cross-sectional area of the rectus capitis posterior minor muscle with active trigger points in chronic tension-type headache: a pilot study. Am J Phys Med Rehabil. 2008; 87:197–203. PMID: 18174844.24. Andary MT, Hallgren RC, Greenman PE, Rechtien JJ. Neurogenic atrophy of suboccipital muscles after a cervical injury: a case study. Am J Phys Med Rehabil. 1998; 77:545–549. PMID: 9862543.25. McPartland JM, Brodeur RR. Rectus capitis posterior minor: a small but important suboccipital muscle. J Bodyw Mov Ther. 1999; 3:30–35.26. Elliott JM, Galloway GJ, Jull GA, Noteboom JT, Centeno CJ, Gibbon WW. Magnetic resonance imaging analysis of the upper cervical spine extensor musculature in an asymptomatic cohort: an index of fat within muscle. Clin Radiol. 2005; 60:355–363. PMID: 15710139.27. Elliott J, Jull G, Noteboom JT, Darnell R, Galloway G, Gibbon WW. Fatty infiltration in the cervical extensor muscles in persistent whiplash-associated disorders: a magnetic resonance imaging analysis. Spine (Phila Pa 1976). 2006; 31:E847–E855. PMID: 17047533.28. Elliott J, Sterling M, Noteboom JT, Darnell R, Galloway G, Jull G. Fatty infiltrate in the cervical extensor muscles is not a feature of chronic, insidious-onset neck pain. Clin Radiol. 2008; 63:681–687. PMID: 18455560.29. Jang JH, Kim TH, Oh JS. Effects of visual display terminal works on cervical movement pattern in patients with neck pain. J Phys Ther Sci. 2014; 26:1031–1032. PMID: 25140089.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The lumbar multifidus is characterised by larger type I muscle fibres compared to the erector spinae
- Clinical Experiences of Trigeminal Rhizotomy using Suboccipital Approach
- Histomorphometry on Regenerated Muscle Fiber in the Damaged Striated Muscle
- Histochemical Muscle Fiber Types of Autopsied Human Gastrocnemius, Soleus, Peroneus longus and Tibialis anterior Muscles
- Histochemical Study of Muscle Fibers in Human Vertebral Muscle