Asia Pac Allergy.
2013 Jan;3(1):59-69. 10.5415/apallergy.2013.3.1.59.
Diagnosis of food allergies: the impact of oral food challenge testing
- Affiliations
-
- 1Department of Allergy, Aichi Children's Health and Medical Center, Aichi 474-8710, Japan. koumei_itoh@mx.achmc.pref.aichi.jp
- KMID: 2397336
- DOI: http://doi.org/10.5415/apallergy.2013.3.1.59
Abstract
- A diagnosis of food allergies should be made based on the observation of allergic symptoms following the intake of suspected foods and the presence of allergen-specific IgE antibodies. The oral food challenge (OFC) test is the most reliable clinical procedure for diagnosing food allergies. Specific IgE testing of allergen components as well as classical crude allergen extracts helps to make a more specific diagnosis of food allergies. The Japanese Society of Pediatric Allergy and Clinical Immunology issued the 'Japanese Pediatric Guideline for Food Allergy 2012' to provide information regarding the standardized diagnosis and management of food allergies. This review summarizes recent progress in the diagnosis of food allergies, focusing on the use of specific IgE tests and the OFC procedure in accordance with the Japanese guidelines.
MeSH Terms
Figure
Cited by 2 articles
-
Evaluation of the results of oral food challenges conducted in specialized and general hospitals
Kazunori Sakai, Kemal Sasaki, Tomoko Furuta, Shiro Sugiura, Yukari Watanabe, Takae Kobayashi, Takashi Kawabe, Masashi Morishita, Kumiko Nakanishi, Komei Ito
Asia Pac Allergy. 2017;7(4):234-242. doi: 10.5415/apallergy.2017.7.4.234.Oral food challenges: result of a 16-year experience at a major teaching hospital in Thailand
Witchaya Srisuwatchari, Pakit Vichyanond
Asia Pac Allergy. 2018;8(2):. doi: 10.5415/apallergy.2018.8.e21.
Reference
-
1. Ebisawa M, Sugizaki C. Prevalence of pediatric allergic diseases in the first 5 years of life. J Allergy Clin Immunol. 2008. 121:S237.
Article2. Umemura H, Kando N, Izumi H, Katou M, Ito K. Evaluation of school lunches of the pediatric patients with food allergies. Jpn J Pediatr Allergy Clin Immunol. 2012. 26:589–598.
Article3. McIntyre CL, Sheetz AH, Carroll CR, Young MC. Administration of epinephrine for life-threatening allergic reactions in school settings. Pediatrics. 2005. 116:1134–1140.
Article4. Borres MP, Ebisawa M, Eigenmann PA. Use of allergen components begins a new era in pediatric allergology. Pediatr Allergy Immunol. 2011. 22:454–461.
Article5. Berni Canani R, Ruotolo S, Discepolo V, Troncone R. The diagnosis of food allergy in children. Curr Opin Pediatr. 2008. 20:584–589.
Article6. García C, El-Qutob D, Martorell A, Febrer I, Rodríguez M, Cerdá JC, Félix R. Sensitization in early age to food allergens in children with atopic dermatitis. Allergol Immunopathol (Madr). 2007. 35:15–20.
Article7. Japanese Society of Pediatric Allergy and Clinical Immunology. Japanese Pediatric Guideline for Oral Food Challenge Test in Food Allergy 2009. 2009. Tokyo: Kyowa-Kikaku.8. Ito K, Urisu A. Diagnosis of food allergy based on oral food challenge test. Allergol Int. 2009. 58:467–474.
Article9. Japanese Society of Pediatric Allergy and Clinical Immunology. Japanese Pediatric Guideline for Food Allergy 2012. 2011. Tokyo: Kyowa-Kikaku.10. Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001. 107:891–896.
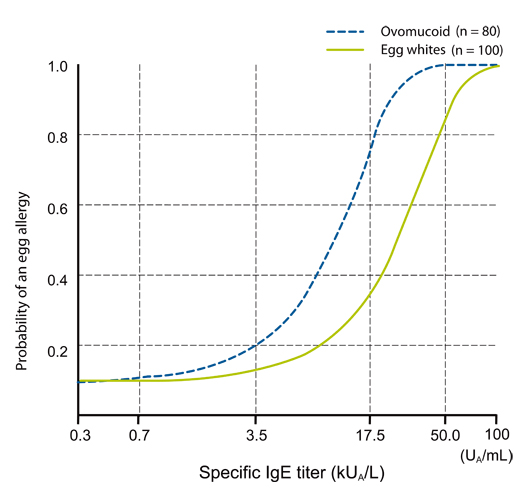
Article11. Urisu A, Ando H, Morita Y, Wada E, Yasaki T, Yamada K, Komada K, Torii S, Goto M, Wakamatsu T. Allergenic activity of heated and ovomucoid-depleted egg white. J Allergy Clin Immunol. 1997. 100:171–176.
Article12. Ando H, Movérare R, Kondo Y, Tsuge I, Tanaka A, Borres MP, Urisu A. Utility of ovomucoid-specific IgE concentrations in predicting symptomatic egg allergy. J Allergy Clin Immunol. 2008. 122:583–588.
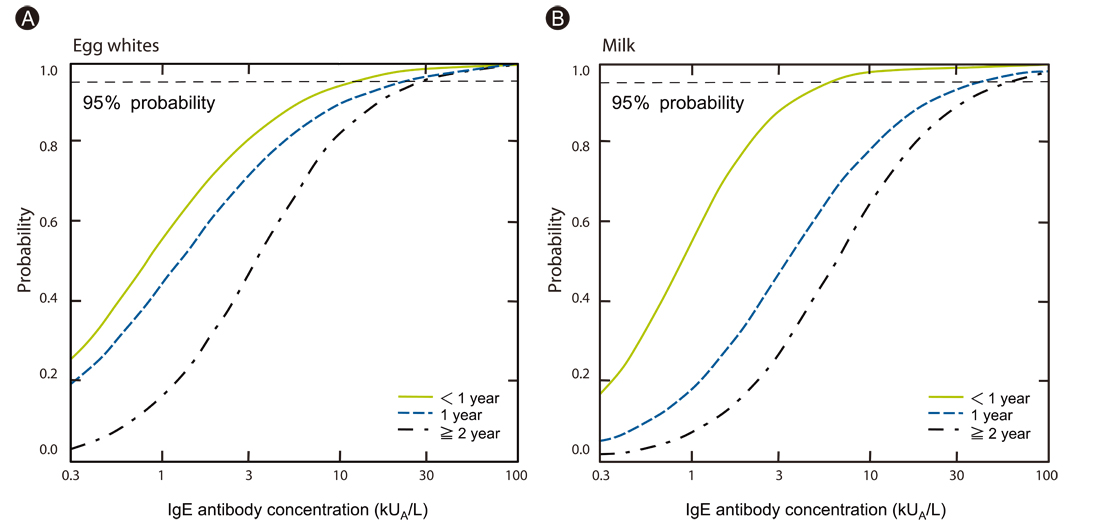
Article13. Komata T, Söderström L, Borres MP, Tachimoto H, Ebisawa M. The predictive relationship of food-specific serum IgE concentrations to challenge outcomes for egg and milk varies by patient age. J Allergy Clin Immunol. 2007. 119:1272–1274.
Article14. Haneda Y, Kando N, Yasui M, Kobayashi T, Maeda T, Hino A, Hasegawa S, Ichiyama T, Ito K. Ovomucoids IgE is a better marker than egg white-specific IgE to diagnose boiled egg allergy. J Allergy Clin Immunol. 2012. 129:1681–1682.
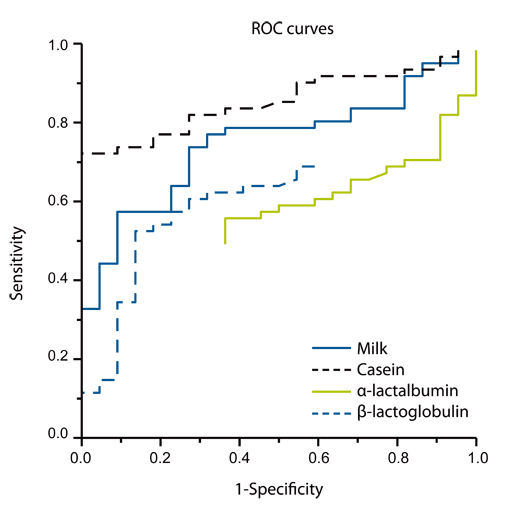
Article15. Ito K, Futamura M, Movérare R, Tanaka A, Kawabe T, Sakamoto T, Borres MP. The usefulness of casein-specific IgE and IgG4 antibodies in cow's milk allergic children. Clin Mol Allergy. 2012. 10:1.
Article16. Urisu A, Yamada K, Masuda S, Komada H, Wada E, Kondo Y, Horiba F, Tsuruta M, Yasaki T, Yamada M, Torii S, Nakamura R. 16-kilodalton rice protein is one of the major allergens in rice grain extract and responsible for cross-allergenicity between cereal grains in the Poaceae family. Int Arch Allergy Appl Immunol. 1991. 96:244–252.
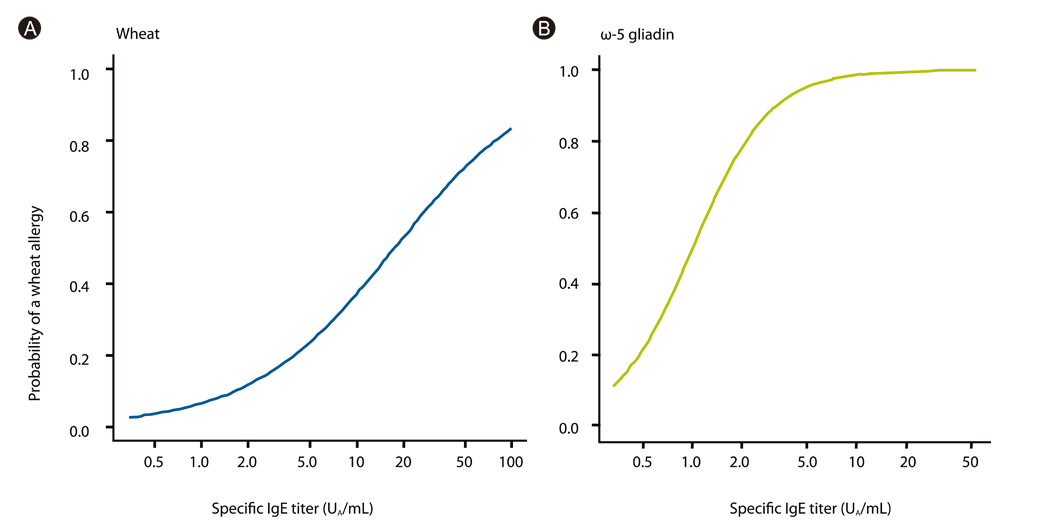
Article17. Ito K, Futamura M, Borres MP, Takaoka Y, Dahlstrom J, Sakamoto T, Tanaka A, Kohno K, Matsuo H, Morita E. IgE antibodies to omega-5 gliadin associate with immediate symptoms on oral wheat challenge in Japanese children. Allergy. 2008. 63:1536–1542.18. Morita E, Matsuo H, Mihara S, Morimoto K, Savage AW, Tatham AS. Fast omega-gliadin is a major allergen in wheat-dependent exercise-induced anaphylaxis. J Dermatol Sci. 2003. 33:99–104.19. Otsuji K, Futamura M, Kando N, Hayashi K, Ito K. Clinical evaluation of ω-5 gliadin-specific IgE test. Arerugi. 2011. 60:971–982.20. Ito K, Morishita M, Ohshima M, Sakamoto T, Tanaka A. Cross-reactive carbohydrate determinant contributes to the false positive IgE antibody to peanut. Allergol Int. 2005. 54:387–392.
Article21. Ebisawa M, Movérare R, Sato S, Maruyama N, Borres MP, Komata T. Measurement of Ara h 1-, 2-, and 3-specific IgE antibodies is useful in diagnosis of peanut allergy in Japanese children. Pediatr Allergy Immunol. 2012. 23:573–581.
Article22. Nowak-Wegrzyn A, Assa'ad AH, Bahna SL, Bock SA, Sicherer SH, Teuber SS. Work Group report: oral food challenge testing. J Allergy Clin Immunol. 2009. 123:S365–S383.23. Rancé F, Deschildre A, Villard-Truc F, Gomez SA, Paty E, Santos C, Couderc L, Fauquert JL, De Blic J, Bidat E, Dupont C, Eigenmann P, Lack G, Scheinmann P. Oral food challenge in children: an expert review. Eur Ann Allergy Clin Immunol. 2009. 41:35–49.24. Niggemann B. Role of oral food challenges in the diagnostic work-up of food allergy in atopic eczema dermatitis syndrome. Allergy. 2004. 59:Suppl 78. 32–34.
Article25. Savage JH, Matsui EC, Skripak JM, Wood RA. The natural history of egg allergy. J Allergy Clin Immunol. 2007. 120:1413–1417.
Article26. Skripak JM, Matsui EC, Mudd K, Wood RA. The natural history of IgE-mediated cow's milk allergy. J Allergy Clin Immunol. 2007. 120:1172–1177.
Article27. Keet CA, Matsui EC, Dhillon G, Lenehan P, Paterakis M, Wood RA. The natural history of wheat allergy. Ann Allergy Asthma Immunol. 2009. 102:410–415.
Article28. Savage JH, Limb SL, Brereton NH, Wood RA. The natural history of peanut allergy: Extending our knowledge beyond childhood. J Allergy Clin Immunol. 2007. 120:717–719.
Article29. Fleischer DM, Conover-Walker MK, Matsui EC, Wood RA. The natural history of tree nut allergy. J Allergy Clin Immunol. 2005. 116:1087–1093.
Article30. Kurihara K. Immunotherapy for food allergy. Allergol Int. 2010. 59:9–14.
Article31. Wood RA. Adkinson NF, Busse WW, Bochner BS, Holgate ST, Simons FE, Lemanske RF, editors. Oral food challenge testing. Middleton's allergy: principles & practice. 2009. Philadelphia: Mosby;1309–1317.32. Perry TT, Matsui EC, Conover-Walker MK, Wood RA. Risk of oral food challenges. J Allergy Clin Immunol. 2004. 114:1164–1168.
Article33. Taylor SL, Hefle SL, Bindslev-Jensen C, Atkins FM, Andre C, Bruijnzeel-Koomen C, Burks AW, Bush RK, Ebisawa M, Eigenmann PA, Host A, Hourihane JO, Isolauri E, Hill DJ, Knulst A, Lack G, Sampson HA, Moneret-Vautrin DA, Rance F, Vadas PA, Yunginger JW, Zeiger RS, Salminen JW, Madsen C, Abbott P. A consensus protocol for the determination of the threshold doses for allergenic foods: how much is too much? Clin Exp Allergy. 2004. 34:689–695.
Article34. Devenney I, Norrman G, Oldaeus G, Strömberg L, Fälth-Magnusson K. A new model for low-dose food challenge in children with allergy to milk or egg. Acta Paediatr. 2006. 95:1133–1139.
Article35. James JM. Respiratory manifestations of food allergy. Pediatrics. 2003. 111:1625–1630.
Article36. Kondo Y, Urisu A. Oral allergy syndrome. Allergol Int. 2009. 58:485–491.
Article37. De Swert LF, Bullens D, Raes M, Dermaux AM. Anaphylaxis in referred pediatric patients: demographic and clinical features, triggers, and therapeutic approach. Eur J Pediatr. 2008. 167:1251–1261.
Article38. Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, Wood RA, Plaut M, Cooper SF, Fenton MJ, Arshad SH, Bahna SL, Beck LA, Byrd-Bredbenner C, Camargo CA Jr, Eichenfield L, Furuta GT, Hanifin JM, Jones C, Kraft M, Levy BD, Lieberman P, Luccioli S, McCall KM, Schneider LC, Simon RA, Simons FE, Teach SJ, Yawn BP, Schwaninger JM. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010. 126:S1–S58.39. Muraro A, Roberts G, Clark A, Eigenmann PA, Halken S, Lack G, Moneret-Vautrin A, Niggemann B, Rancé F. The management of anaphylaxis in childhood: position paper of the European academy of allergology and clinical immunology. Allergy. 2007. 62:857–871.
Article40. Burks AW, Laubach S, Jones SM. Oral tolerance, food allergy, and immunotherapy: implications for future treatment. J Allergy Clin Immunol. 2008. 121:1344–1350.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Food Allergy; Diagnosis and Treatment
- Diagnosis and Treatment of Food Allergy in Children
- Diagnostic Usefulness of the Serum-Specific IgE, the Skin Prick Test and the Atopy Patch Test Compared with That of the Oral Food Challenge Test
- The Diagnosis of Food Allergy in a Pediatric Gastroenterology: Focusing on Non-IgE-mediated Allergic Diseases
- Knowledge and management of food allergy by parents of preschool children who experience food allergies