Yonsei Med J.
2009 Apr;50(2):200-205.
Differential Expression of Activating Transcription Factor-2 and c-Jun in the Immature and Adult Rat Hippocampus Following Lithium-Pilocarpine Induced Status Epilepticus
- Affiliations
-
- 1Department of Neurology, The Catholic University of Korea, Seoul, Korea. nuyikim@catholic.ac.kr
- 2Department of Pharmacology and Neurology, Mayo Clinic, MN, USA.
Abstract
- PURPOSE
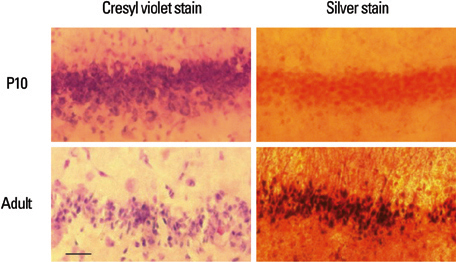
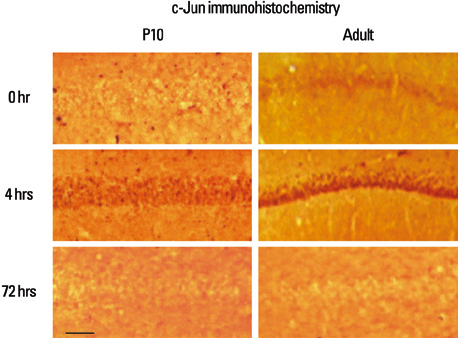
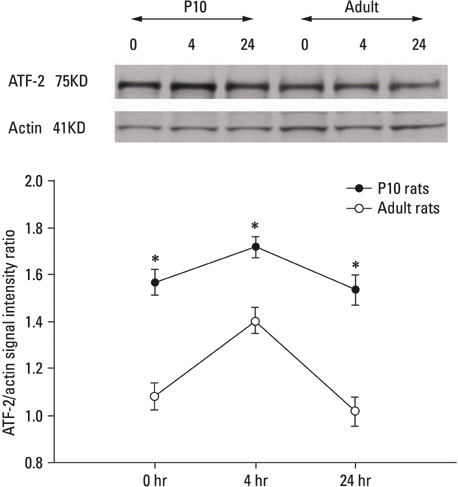
Lithium-pilocarpine induced status epilepticus (LPSE) causes selective and age-dependent neuronal death, although the mechanism of maturation-related injury has not yet been clarified. The activating transcription factor-2 (ATF-2) protein is essential for the normal development of mammalian brain and is activated by c-Jun N-terminal kinase (JNK). It induces the expression of the c-jun gene and modulates the function of the c-Jun protein, a mediator of neuronal death and survival. Therefore, we investigated the expression of c-Jun and ATF-2 protein in the immature and adult rat hippocampus to understand their roles in LPSE-induced neuronal death. MATERIALS AND METHODS: Lithium chloride was administrated to P10 and adult rats followed by pilocarpine. Neuronal injury was assessed by silver and cresyl violet staining, performed 72 hours after status epilepticus. For evaluation of the expression of ATF-2 and c-Jun by immunohistochemical method and Western blot, animals were sacrificed at 0, 4, 24, and 72 hours after the initiation of seizure. RESULTS: Neuronal injury and expression of c-Jun were maturation-dependently increased by LPSE, whereas ATF-2 immunoreactivity decreased in the mature brain. Since both c-Jun and ATF-2 are activated by JNK, and targets and competitors in the same signal transduction cascade, we could speculate that ATF-2 may compete with c-Jun for JNK phosphorylation. CONCLUSION: The results suggested a neuroprotective role of ATF-2 in this maturation-related evolution of neuronal cell death from status epilepticus.
Keyword
MeSH Terms
-
Activating Transcription Factor 2/*metabolism
Animals
Antimanic Agents/pharmacology
Blotting, Western
Hippocampus/drug effects/*metabolism
Immunohistochemistry
Lithium/pharmacology
Miotics/pharmacology
Pilocarpine/pharmacology
Proto-Oncogene Proteins c-jun/*metabolism
Rats
Status Epilepticus/*chemically induced
Figure
Reference
-
1. Dubé C, André V, Covolan L, Ferrandon A, Marescaux C, Nehlig A. C-Fos, Jun D and HSP72 immunoreactivity, and neuronal injury following lithium-pilocarpine induced status epilepticus in immature and adult rats. Brain Res Mol Brain Res. 1998. 63:139–154.
Article2. De Bruin VM, Marinho MM, De Sousa FC, Viana GS. Behavioral and neurochemical alterations after lithium-pilocarpine administration in young and adult rats: a comparative study. Pharmacol Biochem Behav. 2000. 65:547–551.
Article3. Cavalheiro EA, Silva DF, Turski WA, Calderazzo-Filho LS, Bortolotto ZA, Turski L. The susceptibility of rats to pilocarpine-induced seizures is age-dependent. Brain Res. 1987. 465:43–58.
Article4. Priel MR, dos Santos NF, Cavalheiro EA. Developmental aspects of the pilocarpine model of epilepsy. Epilepsy Res. 1996. 26:115–121.
Article5. Ham J, Babij C, Whitfield J, Pfarr CM, Lallemand D, Yaniv M, et al. A c-Jun dominant negative mutant protects sympathetic neurons against programmed cell death. Neuron. 1995. 14:927–939.
Article6. Eilers A, Whitfield J, Babij C, Rubin LL, Ham J. Role of the Jun kinase pathway in the regulation of c-Jun expression and apoptosis in sympathetic neurons. J Neurosci. 1998. 18:1713–1724.
Article7. van Dam H, Wilhelm D, Herr I, Steffen A, Herrlich P, Angel P. ATF-2 is preferentially activated by stress-activated protein kinases to mediate c-jun induction in response to genotixic agents. EMBO J. 1995. 14:1798–1811.
Article8. Gupta S, Campbell D, Dérijard B, Davis RJ. Transcription factor ATF-2 regulation by the JNK signal transduction pathway. Science. 1995. 267:389–393.
Article9. Robinson GA. Changes in the expression of transcription factors ATF-2 and Fra-2 after axotomy and during regeneration in rat retinal ganglion cells. Brain Res Mol Brain Res. 1996. 41:57–64.
Article10. Martin-Villalba A, Winter C, Brecht S, Buschmann T, Zimmermann M, Herdegen T. Rapid and long-lasting suppression of the ATF-2 transcription factor is a common response to neuronal injury. Brain Res Mol Brain Res. 1998. 62:158–166.
Article11. Ferrer I, Blanco R, Carmona M. Differential expression of active, phosphorylation-dependent MAP kinases, MAPK/ERK, SAPK/JNK and p38, and specific transcription factor substrates following quinolinic acid excitotoxicity in the rat. Brain Res Mol Brain Res. 2001. 94:48–58.
Article12. Cheung NS, Carroll FY, Larm JA, Beart PM, Giardina SF. Kainate-induced apoptosis correlates with c-Jun activation in cultured cerebellar granular cells. J Neurosci Res. 1998. 52:69–82.
Article13. Kitayama T, Ogita K, Yoneda Y. Sustained potentiation of AP1 DNA binding is not always associated with neuronal death following systemic administration of kainic acid in murine hippocampus. Neurochem Int. 1999. 35:453–462.
Article14. Dragunow M, Preston K. The role of inducible transcription factors in apoptotic nerve cell death. Brain Res Brain Res Rev. 1995. 21:1–28.
Article15. Gass P, Herdegen T. Neuronal expression of AP-1 proteins in excitotoxic-neurodegenerative disorders and following nerve fiber lesions. Prog Neurobiol. 1995. 47:257–290.
Article16. Someya Y, Inagaki N, Maekawa T, Seino Y, Ishii S. Two 3', 5' - cyclic-adenosine monophosphate response elements in the promoter region of the human gastric inhibitory polypeptide gene. FEBS Lett. 1993. 317:67–73.
Article17. Takeda J, Maekawa T, Sudo T, Seino Y, Imura H, Seito N, et al. Expression of the CRE-BP1 transcriptional regulator binding to the cyclic AMP response element in central nervous system, regenerating liver, and human tumors. Oncogene. 1991. 6:1009–1014.18. Holmes GL. Seizure-induced neuronal injury: animal data. Neurology. 2002. 59(9):Suppl 5. S3–S6.
Article19. Marks JD, Friedman JE, Haddad GG. Vulnerability of CA1 neurons to glutamate is developmentally regulated. Brain Res Dev Brain Res. 1996. 97:194–206.
Article20. Berger ML, Tremblay E, Nitecka L, Ben-Ari Y. Maturation of kainic acid seizure-brain damage syndrome in the rat. III. Postnatal development of kainic acid binding sites in the limbic system. Neuroscience. 1984. 13:1095–1104.
Article21. Sankar R, Shin DH, Wasterlain CG. GABA metabolism during status epilepticus in the developing rat brain. Brain Res Dev Brain Res. 1997. 98:60–64.
Article22. Reimold AM, Grusby MJ, Kosaras B, Fries JW, Mori R, Maniwa S, et al. Chondrodysplasia and neurological abnormalities in ATF-2 deficient mice. Nature. 1996. 379:262–265.
Article23. Kreutz MR, Bien A, Vorwerk CK, Böckers TM, Seidenbecher CI, Tischmeyer W, et al. Co-expression of c-Jun and ATF-2 characterizes the surviving retinal ganglion cells which maintain axonal connections after partial optic nerve injury. Brain Res Mol Brain Res. 1999. 69:232–241.
Article24. Lee JK, Kang SS, Lee MC. Stress protein expression in kainate-induced experimental temporal lobe epilepsy in rats. J Korean Neurosurg Soc. 1998. 27:1641–1652.25. Williams MB, Jope RS. Distinctive rat brain immediate early gene responses to seizures induced by lithium plus pilocarpine. Brain Res Mol Brain Res. 1994. 25:80–89.
Article26. Rikhter TY, Hsu FC, Coulter DA. Gene expression alterations in CA1 area of the rat hippocampus before the onset of epilepsy: A microchip study. 2003. In : AES Proceedings Annual Meeting of the American Epilepsy Society; 2003 Dec 5-10; Boston, Massachusetts. American Epilepsy Society.27. Kim JH, Jung HY, Roh MS, Ahn YM, Kang UG, Kim YS, et al. Developmental Changes in the activation of signal transduction pathway via JNK in rat hippocampus after kainic acid-induced seizure. J Korean Neuropsychiatr Assoc. 2001. 40:971–980.28. Behrens A, Sibilia M, Wagner EF. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat Genet. 1999. 21:326–329.
Article29. Schauwecker PE. Seizure-induced neuronal death is associated with induction of c-Jun N-terminal kinase and is dependent on genetic background. Brain Res. 2000. 884:116–128.
Article30. Yang DD, Kuan CY, Whitmarsh AJ, Rincón M, Zheng TS, Davis RJ, et al. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature. 1997. 389:865–870.
Article31. Lee MC, Rho JL, Kim MK, Woo YJ, Kim JH, Nam SC, et al. c-JUN expression and apoptotic cell death in kainate-induced temporal lobe epilepsy. J Korean Med Sci. 2001. 16:649–656.
Article32. Estus S, Zaks WJ, Freeman RS, Gruda M, Bravo R, Johnso EM Jr. Altered gene expression in neurons during programmed cell death: identification of c-jun as necessary for neuronal apoptosis. J Cell Biol. 1994. 127(6 Pt 1):1717–1727.
Article33. Virgo L, de Belleroche J. Induction of the immediate early gene c-jun in human spinal cord in amyotrophic lateral sclerosis with concomitant loss of NMDA receptor NR-1 and glycine transporter mRNA. Brain Res. 1995. 676:196–204.
Article34. Martín G, Seguí J, Díaz-Villoslada P, Montalbán X, Planas AM, Ferrer I. Jun expression is found in neurons located in the vicinity of subacute plaques in patients with multiple sclerosis. Neurosci Lett. 1996. 212:95–98.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antiepileptic and Neuroprotective Effect of Ketamine in Lithium-Pilocarpine Induced Status Epilepticus Rat Model
- Neuroprotective effect of lithium after pilocarpine-induced status epilepticus in mice
- Time-course changes of hippocalcin expression in the mouse hippocampus following pilocarpine-induced status epilepticus
- Effect of MK-801 on Neuronal Cell Loss and Fos Expression of Hippocampus in Lithium-Pilocarpine Induced Status Epilepticus
- The Effect of Corpus Callosotomy in the Lithium-Pilocarpine Induced Status Epileptic Rats