Korean J Physiol Pharmacol.
2017 Nov;21(6):609-616. 10.4196/kjpp.2017.21.6.609.
Ardipusilloside-I stimulates gastrointestinal motility and phosphorylation of smooth muscle myosin by myosin light chain kinase
- Affiliations
-
- 1College of Pharmacy, Liaoning University of Traditional Chinese Medicine, Liaoning 116600, PR China. huliping705@163.com
- 2College of Information Science & Technology, Liaoning University of Traditional Chinese Medicine, Liaoning 110847, PR China. 826998651@qq.com
- KMID: 2395255
- DOI: http://doi.org/10.4196/kjpp.2017.21.6.609
Abstract
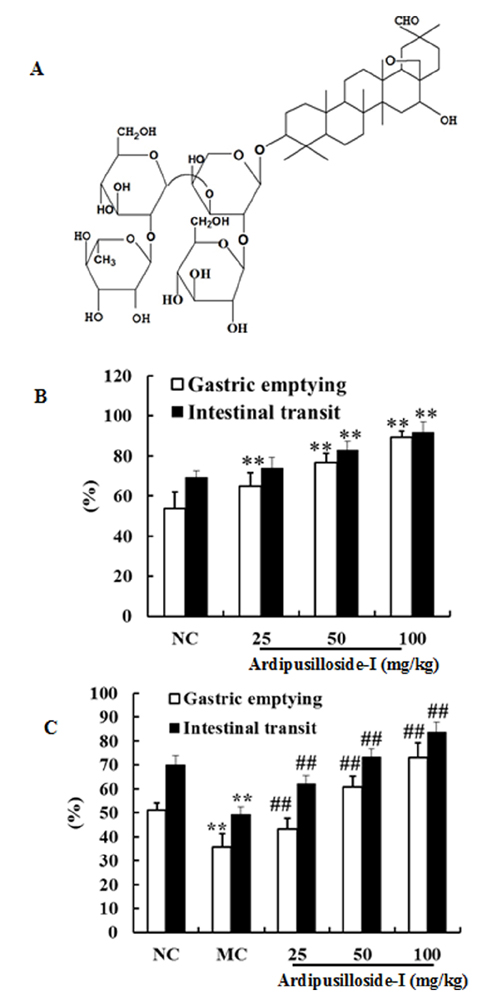
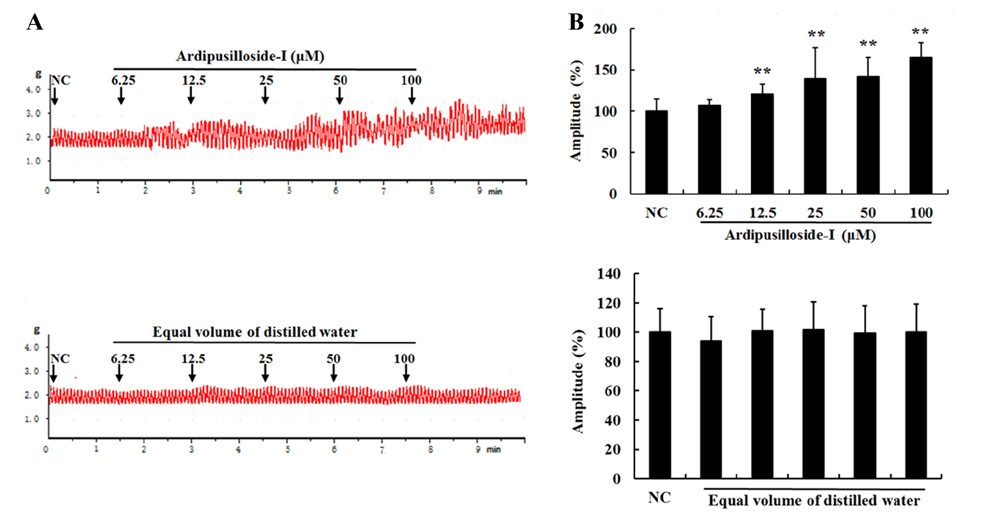
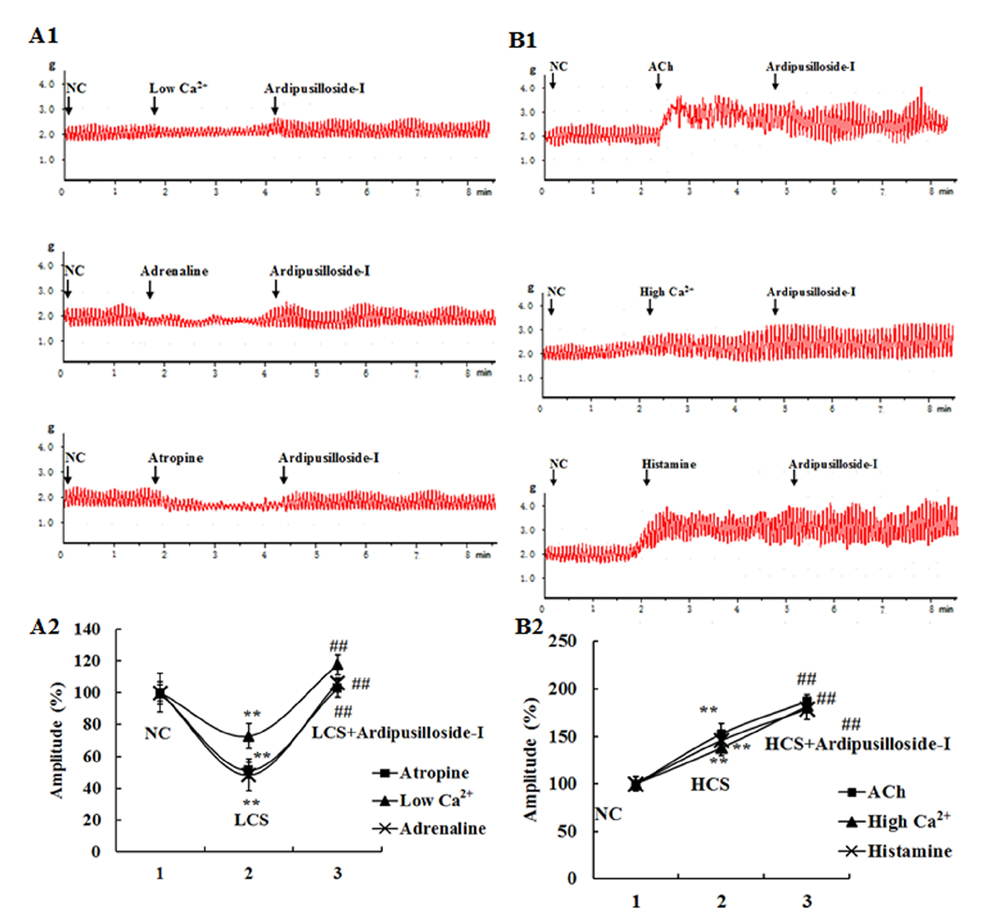
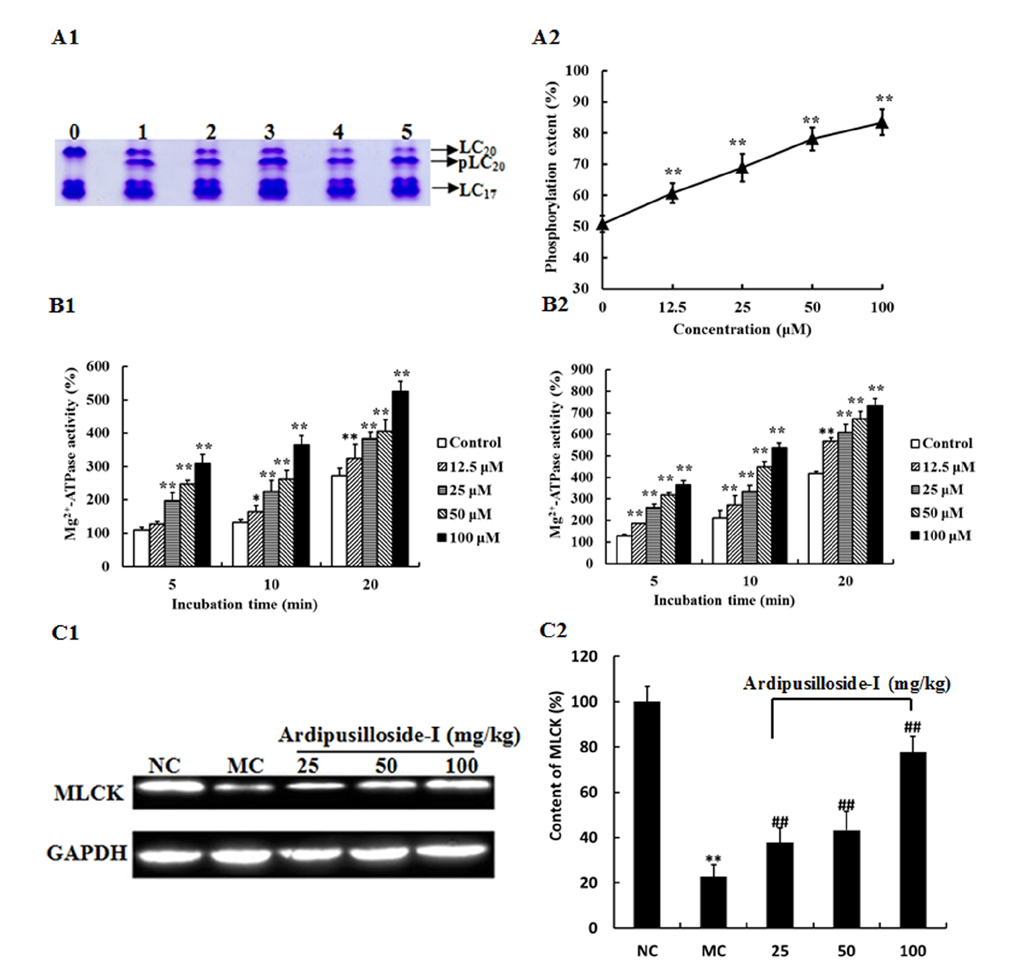
- Ardipusilloside-I is a natural triterpenoid saponin, which was isolated from Ardisia pusilla A. DC. The aim of the study was to evaluate the stimulation of ardipusilloside-I on gastrointestinal motility in vitro and in vivo. The experiment of smooth muscle contraction directly monitored the contractions of the isolated jejunal segment (IJS) in different contractile states, and the effects of ardipusilloside-I on myosin were measured in the presence of Ca²âº-calmodulin using the activities of 20 kDa myosin light chain (MLCâ‚‚â‚€) phosphorylation and myosin Mg²âº-ATPase. The effects of ardipusilloside-I on gastro emptying and intestinal transit in constipation-predominant rats were observed, and the MLCK expression in jejuna of constipated rats was determined by western blot. The results showed that, ardipusilloside-I increased the contractility of IJS in a dose-dependent manner and reversed the low contractile state (LCS) of IJS induced by low Ca²âº, adrenaline, and atropine respectively. There were synergistic effects on contractivity of IJS between ardipusilloside-I and ACh, high Ca²âº, and histamine, respectively. Ardipusilloside-I could stimulate the phosphorylation of MLCâ‚‚â‚€ and Mg²âº-ATPase activities of Ca²âº- dependent phosphorylated myosin. Ardipusilloside-I also stimulated the gastric emptying and intestinal transit in normal and constipated rats in vivo, respectively, and increased the MLCK expression in the jejuna of constipation-predominant rats. Briefly, the findings demonstrated that ardipusilloside-I could effectively excite gastrointestinal motility in vitro and in vivo.
Keyword
MeSH Terms
-
Animals
Ardisia
Atropine
Blotting, Western
Epinephrine
Gastric Emptying
Gastrointestinal Motility*
Histamine
In Vitro Techniques
Muscle, Smooth*
Myosin Light Chains*
Myosin-Light-Chain Kinase*
Myosins*
Phosphorylation*
Rats
Saponins
Atropine
Epinephrine
Histamine
Myosin Light Chains
Myosin-Light-Chain Kinase
Myosins
Saponins
Figure
Reference
-
1. Zhang QH, Huang SL, Wang XJ. Studies on the sapogenins and prosapogenins in Ardisia pusilla A. DC. Zhongguo Zhong Yao Za Zhi. 1993; 18:545–547.2. Cao WY, Wang YN, Wang PY, Lei W, Feng B, Wang XJ. Ardipusilloside-I metabolites from human intestinal bacteria and their antitumor activity. Molecules. 2015; 20:20569–20581.3. Xu XF, Zhang TL, Jin S, Wang R, Xiao X, Zhang WD, Wang PY, Wang XJ. Ardipusilloside I induces apoptosis by regulating Bcl-2 family proteins in human mucoepidermoid carcinoma Mc3 cells. BMC Complement Altern Med. 2013; 13:322.4. Tao XJ, Long JW, He JY, Yao HP, Liu J, Cao YX. Antitumor and immunological regulation effects of ardipusilloside-I. Chinese Pharmacological Bulletin. 2005; 21:1070–1073.5. Vale RD, Milligan RA. The way things move: looking under the hood of molecular motor proteins. Science. 2000; 288:88–95.6. Olson NJ, Pearson RB, Needleman DS, Hurwitz MY, Kemp BE, Means AR. Regulatory and structural motifs of chicken gizzard myosin light chain kinase. Proc Natl Acad Sci U S A. 1990; 87:2284–2288.7. Francis J, Dourish CT, Cooper SJ. Devazepide attenuates dlfenfluramine-induced suppression of gastric emptying but not food intake in the 17 h food-deprived rat. Physiol Behav. 1997; 62:545–550.8. Francis J, Critchley D, Dourish CT, Cooper SJ. Comparisons between the effects of 5-HT and DL-fenfluramine on food intake and gastric emptying in the rat. Pharmacol Biochem Behav. 1995; 50:581–585.9. Trudel L, Tomasetto C, Rio MC, Bouin M, Plourde V, Eberling P, Poitras P. Ghrelin/motilin-related peptide is a potent prokinetic to reverse gastric postoperative ileus in rat. Am J Physiol Gastrointest Liver Physiol. 2002; 282:G948–G952.10. Xu J, Zhou X, Chen C, Deng Q, Huang Q, Yang J, Yang N, Huang F. Laxative effects of partially defatted flaxseed meal on normal and experimental constipated mice. BMC Complement Altern Med. 2012; 12:14.11. Zhu F, Xu S, Zhang Y, Chen F, Ji J, Xie G. Total glucosides of paeony promote intestinal motility in slow transit constipation rats through amelioration of interstitial cells of cajal. PLoS One. 2016; 11:e0160398.12. Yu C, Xiong Y, Chen D, Li Y, Xu B, Lin Y, Tang Z, Jiang C, Wang L. Ameliorative effects of atractylodin on intestinal inflammation and co-occurring dysmotility in both constipation and diarrhea prominent rats. Korean J Physiol Pharmacol. 2017; 21:1–9.13. Lin Y, Ishikawa R, Okagaki T, Ye LH, Kohama K. Stimulation of the ATP-dependent interaction between actin and myosin by a myosin-binding fragment of smooth muscle caldesmon. Cell Motil Cytoskeleton. 1994; 29:250–258.14. Tang ZY, Liu ZN, Fu L, Chen DP, Ai QD, Lin Y. Effect of lithium on smooth muscle contraction and phosphorylation of myosin light chain by MLCK. Physiol Res. 2010; 59:919–926.15. Yang JX, Wang XM, Tang ZY, Chen H, Xu H, Lin Y. The characterization of Ca2+-calmodulin independent phosphorylation of myosin light chains by a fragment from myosin light chain kinase. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai). 2003; 35:793–800.16. Xiong YJ, Chu HW, Lin Y, Han F, Li YC, Wang AG, Wang FJ, Chen DP, Wang JY. Hesperidin alleviates rat postoperative ileus through anti-inflammation and stimulation of Ca2+-dependent myosin phosphorylation. Acta Pharmacol Sin. 2016; 37:1091–1100.17. Webb RC. Smooth muscle contraction and relaxation. Adv Physiol Educ. 2003; 27:201–206.18. Tansey MG, Luby-Phelps K, Kamm KE, Stull JT. Ca2+-dependent phosphorylation of myosin light chain kinase decreases the Ca2+ sensitivity of light chain phosphorylation within smooth muscle cells. J Biol Chem. 1994; 269:9912–9920.19. Gao N, Huang J, He W, Zhu M, Kamm KE, Stull JT. Signaling through myosin light chain kinase in smooth muscles. J Biol Chem. 2013; 288:7596–7605.20. Lin G, Fandel TM, Shindel AW, Wang G, Banie L, Ning H, Lue TF, Lin CS. Modulation of smooth muscle tonus in the lower urinary tract: interplay of myosin light-chain kinase (MLCK) and MLC phosphatase (MLCP). BJU Int. 2011; 108:E66–E70.21. Ammit AJ, Armour CL, Black JL. Smooth-muscle myosin light-chain kinase content is increased in human sensitized airways. Am J Respir Crit Care Med. 2000; 161:257–263.22. He WQ, Peng YJ, Zhang WC, Lv N, Tang J, Chen C, Zhang CH, Gao S, Chen HQ, Zhi G, Feil R, Kamm KE, Stull JT, Gao X, Zhu MS. Myosin light chain kinase is central to smooth muscle contraction and required for gastrointestinal motility in mice. Gastroenterology. 2008; 135:610–620.23. Wang J, Huang JH, Cheng YF, Yang GM. Banana resistant starch and its effects on constipation model mice. J Med Food. 2014; 17:902–907.24. Mostafa SM, Bhandari S, Ritchie G, Gratton N, Wenstone R. Constipation and its implications in the critically ill patient. Br J Anaesth. 2003; 91:815–819.25. Wald A. Chronic constipation: advances in management. Neurogastroenterol Motil. 2007; 19:4–10.26. Xiong YJ, Chen DP, Lv BC, Liu FF, Wang L, Lin Y. Characteristics of nobiletin-induced effects on jejunal contractility. Fitoterapia. 2014; 94:1–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Calcium Sensitization Mechanisms in Gastrointestinal Smooth Muscles
- Pulse exposure to ethanol augments vascular contractility through stress response
- Implication of phosphorylation of the myosin II regulatory light chain in insulin-stimulated GLUT4 translocation in 3T3-F442A adipocytes
- Olanzapine May Inhibit Colonic Motility Associated with the 5-HT Receptor and Myosin Light Chain Kinase
- Ca2+/Calmodulin-Dependent and -Independent Mechanisms are Involved in Angiotension II-induced Contraction of Rat Aortic Smooth Muscle