Cancer Res Treat.
2017 Oct;49(4):960-969. 10.4143/crt.2016.204.
Neurocognitive and Psychological Functioning of Children with an Intracranial Germ Cell Tumor
- Affiliations
-
- 1Proton Therapy Center, National Cancer Center, Goyang, Korea. jooyoungcasa@ncc.re.kr
- 2Department of Radiation Oncology, Soonchunhyang University Seoul Hospital, Seoul, Korea.
- 3Mental Health Clinic, National Cancer Center, Goyang, Korea.
- 4Center for Pediatric Cancer, National Cancer Center, Goyang, Korea.
- KMID: 2394815
- DOI: http://doi.org/10.4143/crt.2016.204
Abstract
- PURPOSE
This study was conducted to investigate the neurocognitive functioning of children with intracranial germ cell tumor (IGCT) prior to receiving proton beam therapy (PBT), and to identify differential characteristics of their neurocognitive functioning depending on tumor location. As a secondary object of this study, neurocognitive functions were followed up at 1-2 years after PBT to examine early post-treatment changes.
MATERIALS AND METHODS
Between 2008 and 2014, 34 childrenwith IGCT treatedwho received PBT atNational Cancer Center, Korea were enrolled in this study. Standardized neurocognitive tests of intelligence, memory, and executive functioning were performed with baseline psychological assessments using the Child Behavior Checklist (CBCL). Follow-up assessments after PBT were conducted in 20 patients (T2). The results were analyzed based on the locations of tumors, which included the suprasellar, pineal gland, basal ganglia, and bifocal regions.
RESULTS
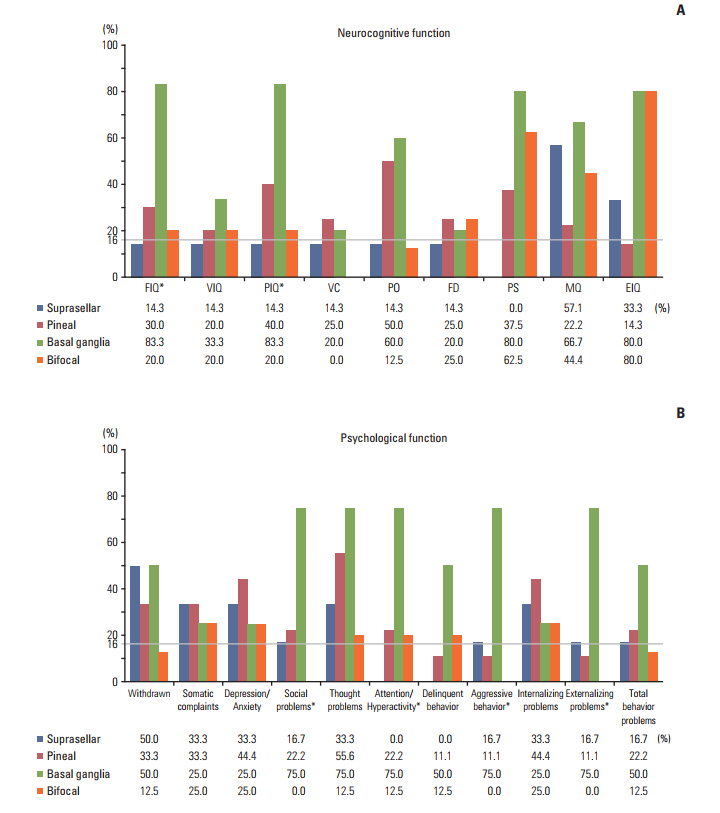
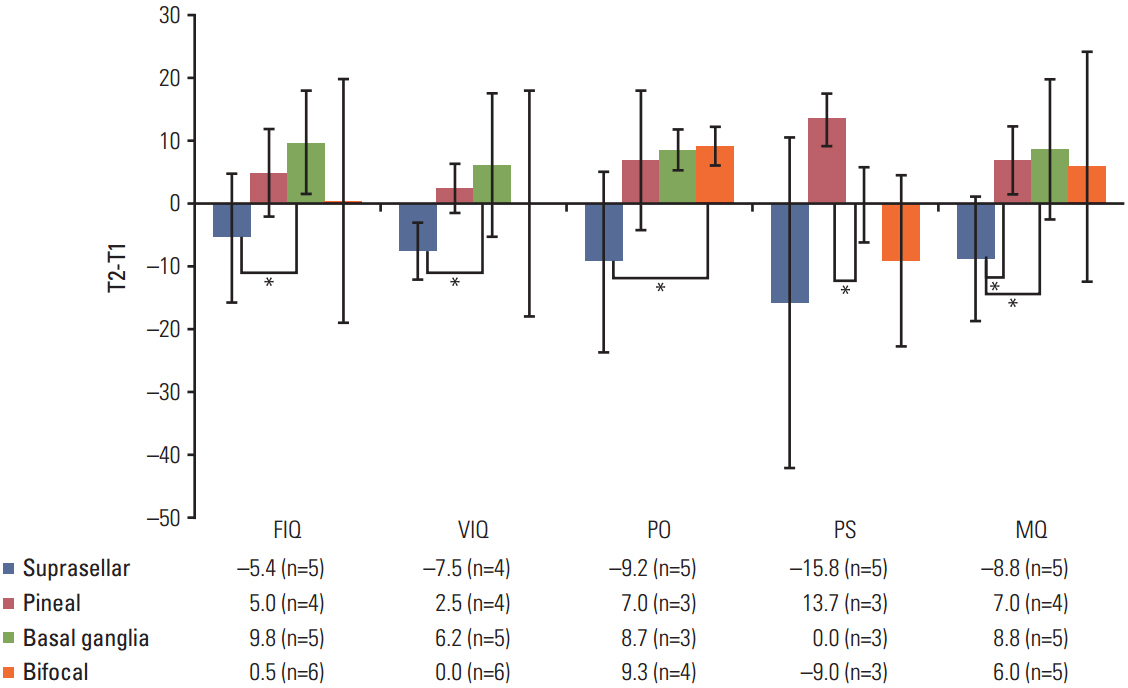
The neurocognitive function of IGCT patients was significantly lower than that of the normal population in performance intelligence quotient (p=0.041), processing speed (p=0.007), memory (p < 0.001), and executive functioning (p=0.010). Patients with basal ganglia tumors had significantly lower scores for most domains of neurocognitive functioning and higher scores for CBCL than both the normal population and patients with IGCT in other locations. There was no significant change in neurocognitive function between T1 and T2 for all types of IGCT patients in first 1-2 years after PBT.
CONCLUSION
Tumor location significantly affects the neuropsychological functioning in patients with IGCT. Neuropsychological functioning should be closely monitored from the time of diagnosis in IGCT patients.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Foo AS, Lim C, Chong DQ, Tan DY, Tham CK. Primary intracranial germ cell tumours: experience of a single South-East Asian institution. J Clin Neurosci. 2014; 21:1761–6.
Article2. Nomura K. Epidemiology of germ cell tumors in Asia of pineal region tumor. J Neurooncol. 2001; 54:211–7.3. Jinguji S, Yoshimura J, Nishiyama K, Aoki H, Nagasaki K, Natsumeda M, et al. Factors affecting functional outcomes in long-term survivors of intracranial germinomas: a 20-year experience in a single institution. J Neurosurg Pediatr. 2013; 11:454–63.
Article4. Merchant TE, Sherwood SH, Mulhern RK, Rose SR, Thompson SJ, Sanford RA, et al. CNS germinoma: disease control and long-term functional outcome for 12 children treated with craniospinal irradiation. Int J Radiat Oncol Biol Phys. 2000; 46:1171–6.
Article5. Schoenfeld A, Haas-Kogan DA, Molinaro A, Banerjee A, Nicolaides T, Tihan T, et al. Pure germinomas of the central nervous system: treatment strategies and outcomes. J Neurooncol. 2014; 120:643–9.
Article6. Kim JY, Baek HJ. The treatment result of intracranial germ cell tumors in Korea: KSPNO G081 multicenter trial, KSPNOG052/082 Trial. New horizon of the germ cell therapy. Seoul: The Korean Society for Pediatric Neuro-Oncology;2013.7. Kim JW, Kim WC, Cho JH, Kim DS, Shim KW, Lyu CJ, et al. A multimodal approach including craniospinal irradiation improves the treatment outcome of high-risk intracranial nongerminomatous germ cell tumors. Int J Radiat Oncol Biol Phys. 2012; 84:625–31.
Article8. Robertson PL, Jakacki R, Hukin J, Siffert J, Allen JC. Multimodality therapy for CNS mixed malignant germ cell tumors (MMGCT): results of a phase II multi-institutional study. J Neurooncol. 2014; 118:93–100.
Article9. Spiegler BJ, Bouffet E, Greenberg ML, Rutka JT, Mabbott DJ. Change in neurocognitive functioning after treatment with cranial radiation in childhood. J Clin Oncol. 2004; 22:706–13.
Article10. Reimers TS, Ehrenfels S, Mortensen EL, Schmiegelow M, Sonderkaer S, Carstensen H, et al. Cognitive deficits in long-term survivors of childhood brain tumors: identification of predictive factors. Med Pediatr Oncol. 2003; 40:26–34.
Article11. Liang SY, Yang TF, Chen YW, Liang ML, Chen HH, Chang KP, et al. Neuropsychological functions and quality of life in survived patients with intracranial germ cell tumors after treatment. Neuro Oncol. 2013; 15:1543–51.
Article12. Wilkening GN, Madden JR. Memory disorders in children with central nervous system germ cell tumors. J Pediatr Oncol Nurs. 2012; 29:161–70.
Article13. Mabbott DJ, Monsalves E, Spiegler BJ, Bartels U, Janzen L, Guger S, et al. Longitudinal evaluation of neurocognitive function after treatment for central nervous system germ cell tumors in childhood. Cancer. 2011; 117:5402–11.
Article14. Iuvone L, Peruzzi L, Colosimo C, Tamburrini G, Caldarelli M, Di Rocco C, et al. Pretreatment neuropsychological deficits in children with brain tumors. Neuro Oncol. 2011; 13:517–24.
Article15. Shortman RI, Lowis SP, Penn A, McCarter RJ, Hunt LP, Brown CC, et al. Cognitive function in children with brain tumors in the first year after diagnosis compared to healthy matched controls. Pediatr Blood Cancer. 2014; 61:464–72.
Article16. Kwak KJ, Park HW, Kim CT. Korean Wechsler Intelligence Scale for Children. 3rd ed. Seoul: Special Education Publishing Co.;2001.17. Yeum TH, Park YS, Oh KJ, Kim JK, Lee YH. The manual of Korean-Wechsler Adult Intelligence Scale. Seoul: Korea Guidance;1992.18. Kim HK. Rey-Kim Memory Test: guidance. Daegu: Neuropsychology Press;2005.19. Kim HK. Kims frontal-executive neuropsychological test. Daegu: Neuropsychology Press;2001.20. Oh K, Lee H. Development of Korean version of Child Behavior Checklist (K-CBCL). Seoul: Korean Research Foundation Report;1990.21. Nathan PC, Patel SK, Dilley K, Goldsby R, Harvey J, Jacobsen C, et al. Guidelines for identification of, advocacy for, and intervention in neurocognitive problems in survivors of childhood cancer: a report from the Children's Oncology Group. Arch Pediatr Adolesc Med. 2007; 161:798–806.22. Nagel BJ, Delis DC, Palmer SL, Reeves C, Gajjar A, Mulhern RK. Early patterns of verbal memory impairment in children treated for medulloblastoma. Neuropsychology. 2006; 20:105–12.
Article23. Jacola LM, Ashford JM, Reddick WE, Glass JO, Ogg RJ, Merchant TE, et al. The relationship between working memory and cerebral white matter volume in survivors of childhood brain tumors treated with conformal radiation therapy. J Neurooncol. 2014; 119:197–205.
Article24. Reimers TS, Mortensen EL, Schmiegelow K. Memory deficits in long-term survivors of childhood brain tumors may primarily reflect general cognitive dysfunctions. Pediatr Blood Cancer. 2007; 48:205–12.
Article25. Wilkening GN, Madden JR, Barton VN, Roberts A, Foreman NK. Memory deficits in patients with pediatric CNS germ cell tumors. Pediatr Blood Cancer. 2011; 57:486–91.
Article26. Williams M, Pennybacker J. Memory disturbances in third ventricle tumours. J Neurol Neurosurg Psychiatry. 1954; 17:115–23.
Article27. Helie S, Chakravarthy S, Moustafa AA. Exploring the cognitive and motor functions of the basal ganglia: an integrative review of computational cognitive neuroscience models. Front Comput Neurosci. 2013; 7:174.
Article28. Leisman G, Braun-Benjamin O, Melillo R. Cognitive-motor interactions of the basal ganglia in development. Front Syst Neurosci. 2014; 8:16.
Article29. Dennis M, Spiegler BJ, Obonsawin MC, Maria BL, Cowell C, Hoffman HJ, et al. Brain tumors in children and adolescents. III. Effects of radiation and hormone status on intelligence and on working, associative and serial-order memory. Neuropsychologia. 1992; 30:257–75.30. Kim JY, Park J. Understanding the treatment strategies of intracranial germ cell tumors: focusing on radiotherapy. J Korean Neurosurg Soc. 2015; 57:315–22.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Characteristics of Intracranial Germ Cell Tumors in Children
- Curatively resected extragonadal germ cell tumor after chemotherapy
- Changes in Endocrine Function with Treatment of Intracranial Germ Cell Tumor
- Cytologic Features of Intracranial Germ Cell Tumors in Crush Preparation
- Understanding the Treatment Strategies of Intracranial Germ Cell Tumors: Focusing on Radiotherapy